
Concept explainers
(a)
Interpretation:
The name of alcohol is to be written and classify as primary, secondary or tertiary alcohol.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(a)
Answer to Problem 42A
Pentanol, primary alcohol
Explanation of Solution
The name of compound is pentanol because it contains five carbon atom and −OH is attached to C-1 of the chain.
1-pentanol is primary alcohol because functional group

(b)
Interpretation:
The name of alcohol is to be written and classify as primary, secondary or tertiary alcohol.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(b)
Answer to Problem 42A
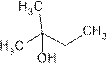
2-Methylbutan-2-ol, tertiary alcohol
Explanation of Solution
The name of compound is2-Methylbutan-2-ol because it contains four carbon atom and one −OH and one methyl is attached to C-2 of the chain.
2-Methylbutan-2-ol is tertiary alcohol because functional group
(c)
Interpretation:
The name of alcohol is to be written and classify as primary, secondary or tertiary alcohol.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(c)
Answer to Problem 42A
Pentan-3-ol, secondary alcohol
Explanation of Solution
The name of compound is pentan-3-ol because it contains five carbon atoms and −OH is attached to C-3 of the chain.
3-pentanol is secondary alcohol because functional group
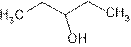
(d)
Interpretation:
The name of alcohol is to be written and classify as primary, secondary or tertiary alcohol.
Concept introduction:
The general formula of alcohol is
The alcohols are named by using suffix ‘ol’.
The alcohols are classified as primary, secondary and tertiary on the basis of nature of carbon atom attached to
If carbon containing
If carbon containing
If carbon containing
(d)
Answer to Problem 42A
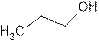
Propanol, primary alcohol
Explanation of Solution
The name of compound is propanol because it contains three carbon atom and −OH is attached to C-1 of the chain.
1-propanol is primary alcohol because functional group
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





