
Concept explainers
(a)
Interpretation:
The structural formula and product for the given reaction should be predicted.
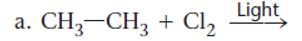
Concept Introduction:
The representation of arrangement of atoms, group of atoms and bonds (double or triple) in the compound is called as structural formula.
(a)
Answer to Problem 64A
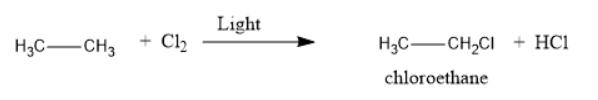
The structural formula and product for the given reaction is represented as follows:
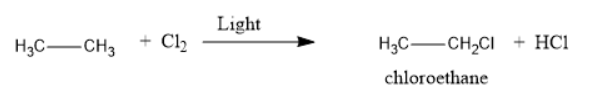
Explanation of Solution
The reaction of
The first step is initiation which involves homolytic cleavage of bond of given molecule giving rise to free radicals on given atom or molecule, second step is propagation in which radicals produced in the first step reacts with given reactant to form a product with a radical on it and attachment of one free radical atom produced in first step. And the third step is termination in which two similar or dissimilar radicals form bonds with each other to form different product.
Thus, the reaction of ethane with chlorine in the presence of light will produce chloroethane along with HCl as a side product.
(b)
Interpretation:
The structural formula and product for the given reaction should be predicted.
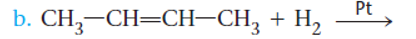
Concept Introduction:
The representation of arrangement of atoms, group of atoms and bonds (double or triple) in the compound is called as structural formula.
(b)
Answer to Problem 64A
The structural formula and product for the given reaction is represented as follows:
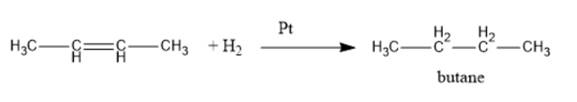
Explanation of Solution
The reaction of an
Thus, but-2-ene on reaction with hydrogen in the presence of platinum leads to the formation of butane. The reaction for the same is represented as follows:
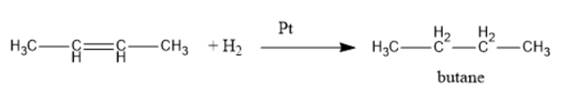
(c)
Interpretation:
The structural formula and product for the given reaction should be predicted.
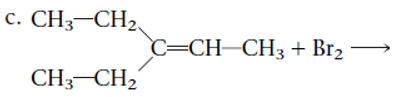
Concept Introduction:
The representation of arrangement of atoms, group of atoms and bonds (double or triple) in the compound is called as structural formula.
(c)
Answer to Problem 64A
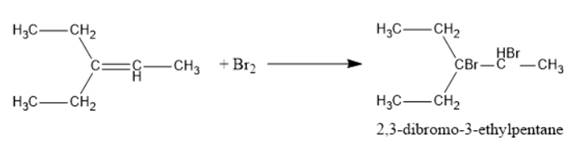
The structural formula and product for the given reaction is represented as follows:
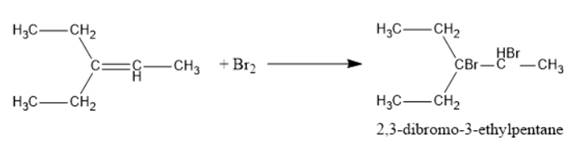
Explanation of Solution
Alkenes undergoes addition reaction due to the pie electron density above and below the plane. Thus, the reaction of given alkene with Br2 gives an addition product and forms 2,3-dibromo-3-ethylpentane. Thus, the
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





