
Concept explainers
a.
Interpretation: The shape of CH4 molecule is to be determined.
Concept Introduction: The shape of a molecule depends on the hybridization of its central atom.
a.
Answer to Problem 2RQ
The shape of CH4 molecule is tetrahedral.
Explanation of Solution
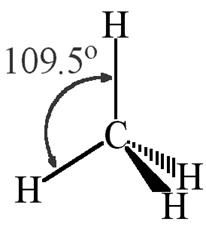
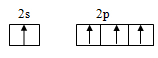
Electronic configuration of carbon is
Ground state configuration:
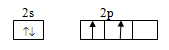
Excited state configuration:
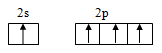
b.
Interpretation: The shape of CH2O molecule is to be determined.
Concept Introduction: The shape of a molecule depends on the hybridization of its central atom.
b.
Answer to Problem 2RQ
The shape of CH2O molecule is trigonal planar.
Explanation of Solution
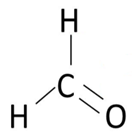
Electronic configuration of carbon is
Ground state configuration:
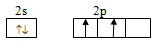
Excited state configuration:
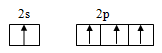
c.
Interpretation: The shape of C2H2 molecule is to be determined.
Concept Introduction: The shape of a molecule depends on the hybridization of its central atom.
c.
Answer to Problem 2RQ
The shape of C2H2 molecule is linear.
Explanation of Solution
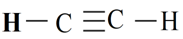
Electronic configuration of carbon is
Ground state configuration:
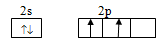
Excited state configuration:
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





