
World of Chemistry
7th Edition
ISBN: 9780618562763
Author: Steven S. Zumdahl
Publisher: Houghton Mifflin College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20, Problem 7STP
Interpretation Introduction
Interpretation: The functional groups present in aspirin needs to be detremined.
Concept introduction:Functional group: An atom or group of atoms in a hydrocarbon derivative that contain elements in addition tocarbon and hydrogen.
Expert Solution & Answer
Answer to Problem 7STP
C. carboxylic acid, ester
Explanation of Solution
The given molecule is aspirin (acetylsalicylic acid) and the structure is as follows:
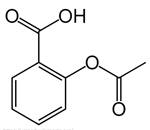
The structure has functional groups -COOH and −COOR, these are the functional groups of carboxylic acid and esters respectively.
Conclusion
Aspirin, an acetyl derivative of salicylic acid, is a white, crystalline, weakly acidic substance.
Chapter 20 Solutions
World of Chemistry
Ch. 20.1 - Prob. 1RQCh. 20.1 - Prob. 2RQCh. 20.1 - Prob. 3RQCh. 20.1 - Prob. 4RQCh. 20.1 - Prob. 5RQCh. 20.1 - Prob. 6RQCh. 20.1 - Prob. 7RQCh. 20.1 - Prob. 8RQCh. 20.2 - Prob. 1RQCh. 20.2 - Prob. 2RQ
Ch. 20.2 - Prob. 3RQCh. 20.2 - Prob. 4RQCh. 20.2 - Prob. 5RQCh. 20.2 - Prob. 6RQCh. 20.3 - Prob. 1RQCh. 20.3 - Prob. 2RQCh. 20.3 - Prob. 3RQCh. 20.3 - Prob. 4RQCh. 20.3 - Prob. 5RQCh. 20.4 - Prob. 1RQCh. 20.4 - Prob. 2RQCh. 20.4 - Prob. 3RQCh. 20.4 - Prob. 4RQCh. 20.4 - Prob. 5RQCh. 20 - Prob. 1ACh. 20 - Prob. 2ACh. 20 - Prob. 3ACh. 20 - Prob. 4ACh. 20 - Prob. 5ACh. 20 - Prob. 6ACh. 20 - Prob. 7ACh. 20 - Prob. 8ACh. 20 - Prob. 9ACh. 20 - Prob. 10ACh. 20 - Prob. 11ACh. 20 - Prob. 12ACh. 20 - Prob. 13ACh. 20 - Prob. 14ACh. 20 - Prob. 15ACh. 20 - Prob. 16ACh. 20 - Prob. 17ACh. 20 - Prob. 18ACh. 20 - Prob. 19ACh. 20 - Prob. 20ACh. 20 - Prob. 21ACh. 20 - Prob. 22ACh. 20 - Prob. 23ACh. 20 - Prob. 24ACh. 20 - Prob. 25ACh. 20 - Prob. 26ACh. 20 - Prob. 27ACh. 20 - Prob. 28ACh. 20 - Prob. 29ACh. 20 - Prob. 30ACh. 20 - Prob. 31ACh. 20 - Prob. 32ACh. 20 - Prob. 33ACh. 20 - Prob. 34ACh. 20 - Prob. 35ACh. 20 - Prob. 36ACh. 20 - Prob. 37ACh. 20 - Prob. 38ACh. 20 - Prob. 39ACh. 20 - Prob. 40ACh. 20 - Prob. 41ACh. 20 - Prob. 42ACh. 20 - Prob. 43ACh. 20 - Prob. 44ACh. 20 - Prob. 45ACh. 20 - Prob. 46ACh. 20 - Prob. 47ACh. 20 - Prob. 48ACh. 20 - Prob. 49ACh. 20 - Prob. 50ACh. 20 - Prob. 51ACh. 20 - Prob. 52ACh. 20 - Prob. 53ACh. 20 - Prob. 54ACh. 20 - Prob. 55ACh. 20 - Prob. 56ACh. 20 - Prob. 57ACh. 20 - Prob. 58ACh. 20 - Prob. 59ACh. 20 - Prob. 60ACh. 20 - Prob. 61ACh. 20 - Prob. 62ACh. 20 - Prob. 63ACh. 20 - Prob. 64ACh. 20 - Prob. 65ACh. 20 - Prob. 66ACh. 20 - Prob. 67ACh. 20 - Prob. 68ACh. 20 - Prob. 1STPCh. 20 - Prob. 2STPCh. 20 - Prob. 3STPCh. 20 - Prob. 4STPCh. 20 - Prob. 5STPCh. 20 - Prob. 6STPCh. 20 - Prob. 7STPCh. 20 - Prob. 8STPCh. 20 - Prob. 9STP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY