
Concept explainers
(a)
Interpretation:
The name of the given molecule is to be determined.
Concept Introduction :
There are various rules for naming the organic compound which are given as,
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest.
- Designate the position of each group with their number and name.
- Collect all the names, listening group alphabetical order by using the full name and use prefix such as di, tri, tetra etc. for the case of several groups of same kind.
(a)
Answer to Problem 8STP
The name is,
Explanation of Solution
Rules for nomenclature for benzene:
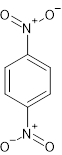
Benzene rings is named on the basis of relative positions of its substituent such as,
Ortho −this prefix is used, if substituent is occupied at the adjacent position.
Meta- This prefix is used, if substituents are separated by one ring position.
Para- this prefix is used, when they are occupying on the opposite side of the ring.
If more than two substitute groups are attached to the ring then the position and number should be mentioned according to the priority order.
There are two nitro groups on opposite carbon of the benzene rings. Thus, name of the compound will be 1, 4-dinitro benzene.
(b)
Interpretation:
The name of the given molecule is to be determined.
Concept Introduction :
There are various rules for naming the organic compound which are given as,
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest.
- Designate the position of each group with their number and name.
- Collect all the names, listening group alphabetical order by using the full name and use prefix such as di, tri, tetra etc. for the case of several groups of same kind.
(b)
Answer to Problem 8STP
The name of the compound is,
Explanation of Solution
The following rules must be followed:
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest.
- Designate the position of each group with their number and name.
The given structure is as follows:
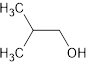
Here, hydroxyl group will get first priority thus, there will be a methyl group at 2nd position.
The name of the compound will be:
(c)
Interpretation:
The name of the given molecule is to be determined.
Concept Introduction :
There are various rules for naming the organic compound which are given as,
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest.
- Designate the position of each group with their number and name.
- Collect all the names, listening group alphabetical order by using the full name and use prefix such as di, tri, tetra etc. for the case of several groups of same kind.
(c)
Answer to Problem 8STP
The given compound is propanal.
Explanation of Solution
There are following rules:
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest
The structure is as follows:
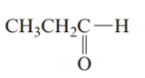
There are 3 carbon atoms in the main chain. Also, there is an
(d)
Interpretation:
The name of the given molecule is to be determined.
Concept Introduction :
There are various rules for naming the organic compound which are given as,
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest.
- Designate the position of each group with their number and name.
- Collect all the names, listening group alphabetical order by using the full name and use prefix such as di, tri, tetra etc. for the case of several groups of same kind.
(d)
Answer to Problem 8STP
The given compound is butanone.
Explanation of Solution
There are following rules:
- Firstly, select the longest continuous chain of carbon atom.
- Secondly, identify the attached functional group and its name also.
- Start the numbering of the chain from the end where a substituent group is nearest
The structure is as follows:
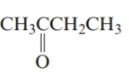
There are 4 carbon atoms in the main chain. Also, there is a
Chapter 20 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





