
Interpretation:
The conversion of D-galactose to D-galacturonic acid is to be suggested, by carrying out specific oxidation.
Concept introduction:
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
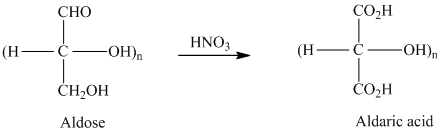
舧 The molecules that are nonsuperimposable or not identical with their mirror images are known as chiral molecules.
舧 A pair of two mirror images that are nonidentical is known as enantiomers, which are optically active.
舧 The stereoisomers that are nonsuperimposable on each other and not mirror images of each other are known as diastereomers.
舧 The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
舧 Compounds that have a plane of symmetry tend to exist in meso forms. A meso form arises when the two stereoisomers produce superimposable images, and hence, compounds having meso forms are optically inactive.
舧 The reaction in which there is removal of water molecule is called dehydration reaction.
舧
舧 Monosaccharide carbohydrates that have their
舧 Based on the given information, direct oxidation of an aldose affects its aldehyde group first, converting it to a carboxylic acid. Most oxidizing agents that attack
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- The reaction of ninhydrin with an -amino acid occurs in several steps (a) The first step is loss of water to give a triketone. Show the mechanism of the reaction and the structure of the triketone.(b) The second step is formation of an imine by reaction of the amino acid with the triketone. Show its structure. (c) The third step is a decarboxylation. Show the structure of the product and the mechanism of the decarboxylation reaction. (d) The fourth step is hydrolysis of an imine to yield an amine and an aldehyde. Show the structures of both products. (e) The final step is formation of the purple anion. Show the mechanism of the reactionarrow_forwardExplain the products that would be formed when cholesterol is reacted with bromine in an organic solvent. Outline a mechanism for the reaction that takes place.arrow_forwardd-Glucuronic acid is found widely in plants and animals. One of its functions is to detoxify poisonous HO-containing compounds by reacting with them in the liver to form glucuronides. Glucuronides are water soluble and, therefore, readily excreted. After ingestion of a poison such as turpentine or phenol, the glucuronides of these compounds are found in urine. Draw the structure of the glucuronide formed by the reaction of beta-d-glucuronic acid and phenol.arrow_forward
- When the gum of the shrub Sterculia setigera is subjected to acidic hydrolysis, one of the water-soluble components of thehydrolysate is found to be tagatose. The following information is known about tagatose:(1) Molecular formula C6H12O6(2) Undergoes mutarotation.(3) Does not react with bromine water.(4) Reduces Tollens reagent to give d-galactonic acid and d-talonic acid.(5) Methylation of tagatose (using excess CH3 I and Ag2O) followed by acidic hydrolysis gives1,3,4,5-tetra-O-methyltagatose.(a) Draw a Fischer projection structure for the open-chain form of tagatose.(b) Draw the most stable conformation of the most stable cyclic hemiacetal form of tagatosearrow_forward(a) Which of the d-aldopentoses will give optically active aldaric acids on oxidation with HNO3 ?(b) Which of the d-aldotetroses will give optically active aldaric acids on oxidation with HNO3 ?(c) Sugar X is known to be a d-aldohexose. On oxidation with HNO3, X gives an optically inactive aldaric acid. WhenX is degraded to an aldopentose, oxidation of the aldopentose gives an optically active aldaric acid. Determine thestructure of X.(d) Even though sugar X gives an optically inactive aldaric acid, the pentose formed by degradation gives an opticallyactive aldaric acid. Does this finding contradict the principle that optically inactive reagents cannot form opticallyactive products?(e) Show what product results if the aldopentose formed from degradation of X is further degraded to an aldotetrose.Does HNO3 oxidize this aldotetrose to an optically active aldaric acid?arrow_forwardprovide the structure for the product followin gthe reaction sequencearrow_forward
- A is a key intermediate in an enzyme mediated reaction. A immediately reacts further to give B and C. Provide mechanisms to explain the formation of B and C. The reaction takes place in H2O (solvent).arrow_forwardThoroughly explain why (a)malthose is a reducing sugar while trehalose is not based on their structures. (b)Why is trehalose very resistant to acid hydrolysis while maltose can be acid-hydrolyzed with ease. Give clear explanations.arrow_forwardAcetic anhydride is hydrolyzed by water to provide two molecules of acetic acid. Propose amechanism for this reaction.arrow_forward
- Consider the synthetic sequence shown. Identify the reagents for all three steps. Draw the structures of organic compounds A and B. Omit byproducts.arrow_forwardTreatment of cholesterol with mCPBA results in formation of a single epoxide A, with the stereochemistry drawn. Why isn’t the isomeric epoxide B formed to any extent?arrow_forwardShow how you will useModified Gabriel’s Synthesis and Streckers’s Synthesis to prepare phenylalanine in the laboratory.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

