
Concept explainers
Interpretation:
The distinguishing tests for the pair of given compounds are to be determined.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
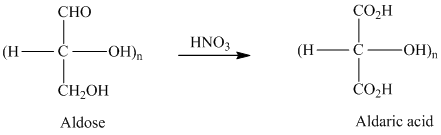
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 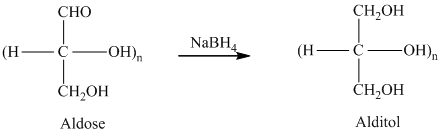
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 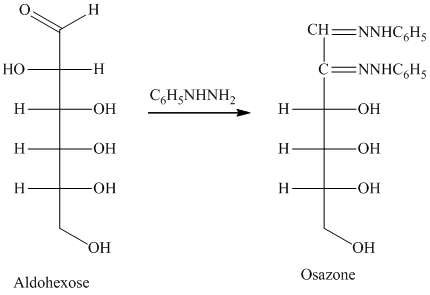
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧. Bromine water is an effective reagent that selectively oxidizes the
舧 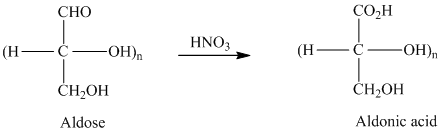
Tollen’s (
舧 Compounds that have plane of symmetry tend to form meso compounds. A meso form arises when the two stereoisomers produce superimposable (achiral) images, and hence, compounds having meso are optically inactive. Chiral (or non-superimposable) molecules are optically active.
舧
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- How many asymmetric carbons and stereoisomers are there for an aldohexose? For aketohexose?arrow_forwardA reddish color is obtained when compound A (a disaccharide) is reacted with Benedict solution. Is this compound more likely maltose or sucrosearrow_forwardProvide an explanation for the fact that α-D-mannose is more stable thanβ-D-mannose, whereas the opposite is true for glucose.arrow_forward
- The anticoagulant heparin is a polysaccharide that contains alternating residues of -D- glucuronic acid-6- sulfate and N-sulfo-D-glucosamine-6sulfate connected by (1 B 4)- glycosidic linkages. Draw a part of heparin that shows one each of the two residues.arrow_forwardA second category of six-carbon carbohydrates, called ketohexoses, has the constitution shown. How many stereoisomeric 2-ketohexoses are possible?arrow_forwardDraw and identify the structuresof glucose, its anomers, and itsepimers,arrow_forward
- 1. Identify the anomeric carbons on sucrose and explain how they are different from lactose and cellulose. 2. On Wikipedia (image pictures below) sucrose is labeled β-D-Fructofuranosyl α-D-glucopyranoside. But the anomeric carbon has the oxygen pointing down and other stereocenters inverted from that of fructose. Did Wikipedia incorrectly name it? Or can you explain why this is the case using structures of sucrose, fructose and glucose…arrow_forwardHow many constitutional isomers are possible for a triglyceride containing one molecule each of palmitic acid, oleic acid, and stearic acid? (b) Which of these constitutional isomers are chiral?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT





