
Concept explainers
Interpretation:
The structures of the two different
Concept introduction:
舧 A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen-oxygen atom ratio of 2:1
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
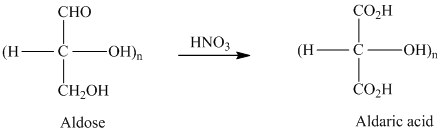
舧
舧 Aldaric acids (obtained from oxidation of aldohexoses) produces lactones of the same order that is as:
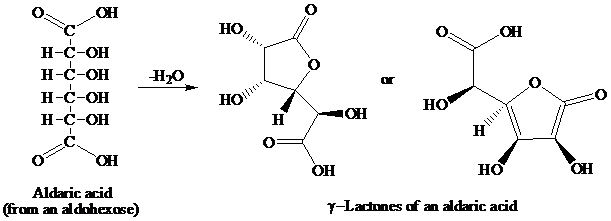
舧 The reaction in which there is removal of water molecule is called dehydration reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Identify the hemiacetal function in lactose and show mechanistically how the stereochemistry at that carbon can easily invert, especially in the presence of acids and bases.arrow_forwardWolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forwardCompound A is a branched-chain alcohol that undergoes oxidation to produce compound B. Compound B is a ketone that gives positive triiodomethane reaction. Compound B is then reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueous acid to form compound C. Compound C has the molecular formula of C11H16O (i) Deduce the structure for compound A, B and C. (ii) State the observation when compound C is added with acidified potassium dichromate(VI).arrow_forward
- Treatment of salicylaldehyde (2-hydroxybenzaldehyde) with bromine in glacial acetic acid at 0°C gives a compound with the molecular formula C7H4Br2O2, which is used as a topical fungicide and antibacterial agent. Propose a structural formula for this compoundarrow_forwardCompound J, C16H16Br2, is optically active. On treatment with strong base, compounds K and L (each C16H14) are formed; K and L each absorb only 2 equivalents of hydrogen when reduced over a Pd/C catalyst. Compound K reacts with ozone to give phenylacetic acid (C6H5CH2COOH), while similar treatment of L gives 2 products. One product, M, is an aldehyde with formula C7H6O; the other product is glyoxal (CHO)2. Draw the structure of compound L.arrow_forwardCompound A has the molecular formula C14H25Br and was obtained by reaction of sodium acetylide (HC≡CNa) )with 1,12-dibromododecane. On treatment of compound A with sodium amide, it was converted to compound B (C14H24). Ozonolysis of compound B gave the diacid HO2C(CH2)12CO2H. Catalytic hydrogenation of compound B over Lindlar palladium gave compound C (C14H26), while hydrogenation over platinum gave compound D (C14H28). Sodium-ammonia reduction of compound B gave compound E (C14H26). Both C and E yielded O═CH(CH2)12CH═O on ozonolysis. Assign structures to compound A through E so as to be consistent with the observed transformations.arrow_forward
- Outline the theoretically possible stereochemical outcomes of reduction of benzil. Describe isomers of hydrobenzoin and stereochemical relationships between them.arrow_forwardDraw the structure of sodium tetradecyl sulfate and ciprofloxacin and state how the activity of the drug is influenced by alkane groupings.arrow_forwardCompound A has the molecular formula C14H25Br and was obtained by thereaction of sodium acetylide with 1,12-dibromododecane. On treatment ofcompound A with sodium amide, compound B (C14H24) was obtained. Ozonolysisof compound B gave the diacid HO2C(CH2)12CO2H. Catalytic hydrogenation ofcompound B over Lindlar palladium gave compound C (C14H26), andhydrogenation over platinum gave compound D (C14H28). Further, C yieldedO=CH(CH2)12CH=O on ozonolysis. Assign structures to compounds A through Dso as to be consistent with the observed transformations.arrow_forward
- Compound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forwardA chiral amine A having the R configuration undergoes Hofmann elimination to form an alkene B as the major product. B is oxidatively cleaved with ozone, followed by CH3SCH3, to form CH2 = O and CH3CH2CH2CHO. What are the structures of A and B?arrow_forwardBriefly outline how the enantiomers of phenylsuccinic acid will be separated from each other, starting from racemic phenylsuccinic acid to isolating (+)-phenylsuccinic acid. Explain the purpose of each step.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


