
Concept explainers
Interpretation:
The names and structures of the aldohexoses based on the results of the products obtained through the reaction of these aldohexoses with phenyl hydrazine and hydrogen (in presence of catalyst) are to be given.
Concept introduction:
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
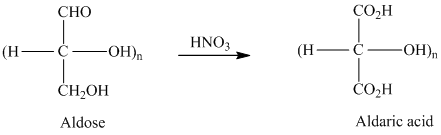
舧 Monosaccharides containing six carbon atoms and an
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 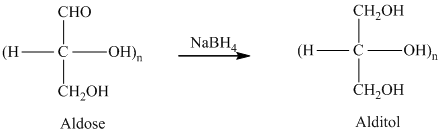
Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 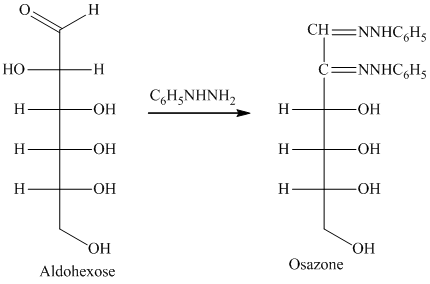
Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Quinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forward(a) Which of the d-aldopentoses will give optically active aldaric acids on oxidation with HNO3 ?(b) Which of the d-aldotetroses will give optically active aldaric acids on oxidation with HNO3 ?(c) Sugar X is known to be a d-aldohexose. On oxidation with HNO3, X gives an optically inactive aldaric acid. WhenX is degraded to an aldopentose, oxidation of the aldopentose gives an optically active aldaric acid. Determine thestructure of X.(d) Even though sugar X gives an optically inactive aldaric acid, the pentose formed by degradation gives an opticallyactive aldaric acid. Does this finding contradict the principle that optically inactive reagents cannot form opticallyactive products?(e) Show what product results if the aldopentose formed from degradation of X is further degraded to an aldotetrose.Does HNO3 oxidize this aldotetrose to an optically active aldaric acid?arrow_forwardThe cyclic hemiacetal is more stable than the open-chain form, so very little of the open-chain form is present atequilibrium. Will an aqueous solution of glucose reduce Tollens reagent and give a positive Tollens test? Explain.arrow_forward
- A chiral amine A having the R conguration undergoes Hofmann elimination to form an alkene B as the major product. B is oxidatively cleaved with ozone, followed by CH3SCH3, to form CH2 = O and CH3CH2CH2CHO. What are the structures of A and B?arrow_forwardUnder the Classification of Mechatronic Products, provide at least 2 examples for each class and explain why such product belongs to a particular class.arrow_forwardThe ketone shown was prepared in a three-step sequence from ethyl trifluoroacetate. The first step in the sequence involved treating ethyl trifluoroacetate with ammonia to give compound A. Compound A was in turn converted to the desired ketone by way of compound B. Fill in the missing reagents in the sequence shown, and give the structures of compounds A and B.arrow_forward
- The following isomerization reaction, drawn using D-glucose as starting material, occurs with all aldohexoses in the presence of base. Draw a stepwise mechanism that illustrates how each compound is formed.arrow_forwardChoose the product that is expected when the β-pyranose form of compound A is treated with excess ethyl iodide in the presence of silver oxide. The following information can be used to determine the identity of compound A: 1. The molecular formula of compound A is C6H12O6.2. Compound A is a reducing sugar.3. When compound A is subjected to a Wohl degradation two times sequentially, D-erythrose is obtained.4. Compound A is epimeric with D-glucose at C3.5. The configuration at C2 is R.arrow_forwardWhat product is formed when each compound undergoes anintramolecular reaction in the presence of acid?Hydroxy aldehydes A and B readily cyclize to form hemiacetals. Draw thestereoisomers formed in this reaction from both A and B. Explain whythis process gives an optically inactive product mixture from A and anoptically active product mixture from B.arrow_forward
- Aldohexoses A and B both undergo Ruff degradation to give aldopentose C. On treatment with warm nitric acid, aldopentose C gives an optically active aldaric acid. B alsoreacts with warm nitric acid to give an optically active aldaric acid, but A reacts to givean optically inactive aldaric acid. Aldopentose C is degraded to aldotetrose D, whichgives optically active tartaric acid when it is treated with nitric acid. Aldotetrose D isdegraded to (+)@glyceraldehyde. Deduce the structures of sugars A, B, C, and D, and useFigure 23-3 to determine the correct names of these sugars.arrow_forwardFollowing are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forwardTreatment of α,β-unsaturated carbonyl compound X with base forms the diastereomer Y. Write a stepwise mechanism for this reaction. Explain why one stereogenic center changes configuration but the other does not.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

