
Concept explainers
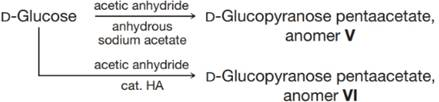
The
(a) Which proton in these anomers would be expected to have these highest
(b) Why do the signals for these protons appear as doublets?
(c) The relationship between the magnitude of the observed coupling constant and the dihedral angle (when measured using a Newman projection) between C−-H bonds on the adjacent carbons of a C−-C bond is given by the Karplus equation. It indicates that an axial–axial relationship results in a coupling constant of about 9 Hz (observed range is 8–14 Hz) and an equatorial–axial relationship results in a coupling constant of about 2 Hz (observed range is 1–7 Hz). Which of V and VI is the αa anomer and which is the
(d) Draw the most stable conformer for each of V and VI.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
Additional Science Textbook Solutions
CHEMISTRY-TEXT
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Chemistry: A Molecular Approach (4th Edition)
Elementary Principles of Chemical Processes, Binder Ready Version
Living by Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
- ) Why do most long-chain fatty acids show a large peak in the mass spectrum at m>z 60?arrow_forwardIR spectroscopy played a key role in elucidating the structure of penicillin G. Explain why the carbonyl on β-Lactams absorbs at ~1760 cm −1, which is much higher than observed in most of other amides.arrow_forwardWhich of the following structures has the highest resolution? a. An X-ray structure with 3.0 Ǻ resolution b. An X-ray structure with 4.0 Ǻ resolution c. An X-ray structure with 1.5 Ǻ resolution d. An X-ray structure with 1.0 Ǻ resolution e. An NMR structure with 10 NOE’sarrow_forward
- Describe the 1H NMR peak caused by this specific hydrogen on cholesterol.arrow_forwardDetermine the most likely structure of a compound with the formula C9H12 if it gave an H NMR spectrum consisting of: A doublet at d 1.25, a septet at d 2.90 and a multiplex at d 7.25arrow_forwarddrawings of the 1H-NMR and 13C-NMR spectrums for this lysine?arrow_forward
- give structure of this c-nmr spectrumarrow_forwardWhich of the following techniques will confirm that theconstitutional isomers propyl benzene and isopropylbenzene are structurally different?a. FTIRb.1H NMRc. 23Na NMRd. UV/VISarrow_forwardWhat are the functional groups in Camphor and Isoborneol? What peaks show up in their respective IR spectrum + wavenumber (cm-1)arrow_forward
- What's the splitting pattern of the the bold carbon in C13-NMR? ( CH3)2CHCH2CH2OHarrow_forwardWhen the 1HNMR spectrum of an alcohol is run in dimethylsulfoxide (DMSO) solvent rather than in chloroform, exchange of the Ο-H proton is slow and spin-spin splitting is seen between the Ο-H proton and C-H protons on the adjacent carbon. What spin multiplicities would you expect for the hydroxyl protons in the following alcohols? (a) 2-Methyl-2-propanol (b) Cyclohexanol (c) Ethanol (d) 2-Propanol (e) Cholesterol (f) 1-Methylcyclohexanolarrow_forwardThe following data was collected for this experiment: A sample of 0.8281 g of phenylsuccinic acid was dissolved in 10 mL of acetone. This sample gave a reading, aobs, of +10.278 deg on the polarimeter. A tube measuring 1 dm was used for the sample. What is the major enantiomer present in this sample? Calculate the concentration of the sample used in g/mL. Calculate the observed specific rotation [a]obs of the sample. Give your answer to 4 significant figures.arrow_forward

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

