
Concept explainers
Interpretation:
The structure of lactose sugar based on the given experimental evidences is to be shown.
Concept introduction:
舧 Chair conformations: It is the most stable conformation, which accurately shows the spatial arrangement of atoms.
舧 Equatorial bonds are parallel to the average plane of the ring, while axial bonds are perpendicular to the average plane of the ring.
舧 The conformation having bonds at the equatorial positions are more stable than those with bonds at the axial position.
舧 On flipping the cyclohexane ring, axial bonds become equatorial bonds and equatorial bonds becomes axial bond.
舧 Bulkier group acquires equatorial positions to form stable conformer due to steric factors.
舧 The most stable configuration of aldopyranoses is when the
舧 Stereochemistry: The equatorial orientation refers to the spatial arrangement of
舧 The anomeric effect is lowest for sugars with equatorial orientation, which results in lower energetic state, and consequently this type of orientation confers higher stability.
舧 The anomeric effect is highest for sugars with axial orientation, which results in higher energetic state, and consequently this type of orientation confers lower stability.
舧 A carbohydrate is a
舧
舧 Carbohydrates are oxidized by
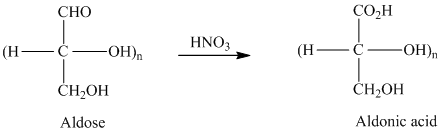
舧 Aldaric acids are carbohydrates having two carboxylic acids. They are formed due to oxidation reaction of aldoses with dilute
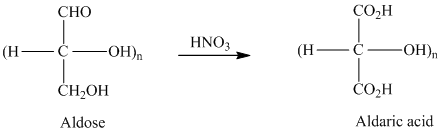
舧 Monosaccharides containing six carbon atoms and an aldehyde group are called aldohexoses.
舧 Alditols are compounds produced from aldoses or ketoses on reduction with certain reagents such as sodium borohydride (
舧 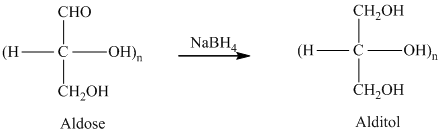
舧 Compounds formed by the reaction of reducing sugars with excess of phenyl hydrazine are called osazones. Osazones are products of oxidation and are produced by all reducing sugars.
舧 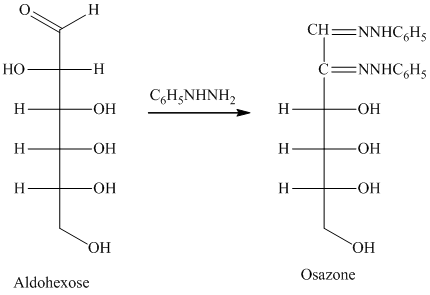
舧 Fischer projection is a way of representing the structural formulae of compounds through cross formulation of their open chain structures.
舧 Bromine water is an effective reagent that selectively oxidizes the
舧 Lactose is a disaccharide carbohydrate present in milk (mammary glands) of the mammals. It has a reducing nature and undergoes hydrolysis to yield

舧 The aldehyde group of an aldose reacts with three moles of phenylhydrazine to produce phenylosazone at
舧 The monosaccharides with five or more carbons atoms form cyclic structures in aqueous solution, and they can form two cyclic stereoisomers from each straight-chain monosaccharide, known as anomers. In aqueous solution, the two anomers and the straight-chain structure of a monosaccharide form an equilibrium mixture through the process of mutarotation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Draw the structure of leu-enkephalin, a pentapeptide that acts as an analgesic and opiate, and has the following sequence: Tyr–Gly–Gly–Phe–Leu. (The structure of a related peptide, met-enkephalin, appeared in Section 22.6B.)arrow_forwardTrehalose is a nonreducing disaccharide (C12H22O11) isolated from the poisonous mushroom Amanita muscaria. Treatment with an a@glucosidase converts trehalose to two molecules of glucose, but no reaction occurs when trehalose is treated with a b@glucosidase.When trehalose is methylated by dimethyl sulfate in mild base and then hydrolyzed,the only product is 2,3,4,6-tetra-O-methylglucose. Propose a complete structure andsystematic name for trehalose.arrow_forwardVanillin (4-hydroxy-3-methoxybenzaldehyde), the principal component of vanilla, occurs in vanilla beans and other natural sources as a b-d-glucopyranoside. Draw a structural formula for this glycoside, showing the d-glucose unit as a chair conformation.arrow_forward
- Cystine is an amino acid formed from the oxidation of twocysteine molecules to form a disulfide bond. The molecular formula of cystine is C6H12O4N2S2. Draw the structural formula of cystine. (arrow_forwardA graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active. Does the product have any asymmetric carbon atoms or other stereocenters?arrow_forwardC6 H 1206 is the chemical formula for a polymer of carbohydrate pentose monosaccharide hexose monosaccharide or allarrow_forward
- How much lactic acid (C3H6O3) is produced when 225g glucose (C6H12O6) is used as a substrate in fermentation. Assuming that the sugar is completely fermented to lactic acid.arrow_forwardXylulose has the following structural formula. To what carbohydrate class does xylulose belong based on the number of carbons and carbonyl functionality? A) aldotetrose B) aldopentose C) ketotetrose D) ketopentose E) ketohexosearrow_forwardGive the major organic product that is formed when the primary hydroxyl group of the following monosaccharide undergo nucleophilic substitution reaction with acetic anhydridearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


