
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 16PS
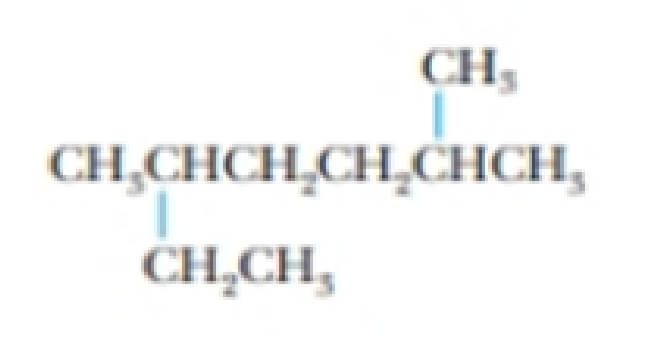
Give the systematic name for the following
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 23 Solutions
Chemistry & Chemical Reactivity
Ch. 23.2 - (a) Draw the nine isomers having the formula...Ch. 23.2 - Prob. 23.2CYUCh. 23.2 - There are 17 possible alkene isomers with the...Ch. 23.2 - Prob. 23.4CYUCh. 23.2 - Aniline, C6H5NH2, is the common name for...Ch. 23.3 - Draw the structure of 1-butanol and alcohols that...Ch. 23.4 - (a) Name each of the following compounds and its...Ch. 23.5 - Kevlar is a well-known polymer that is now used to...Ch. 23.5 - Prob. 1.1ACPCh. 23.5 - Prob. 1.2ACP
Ch. 23.5 - Prob. 1.3ACPCh. 23.5 - Prob. 2.1ACPCh. 23.5 - Prob. 2.2ACPCh. 23.5 - Prob. 2.3ACPCh. 23.5 - What is the atom economy for the reaction of...Ch. 23.5 - Prob. 3.2ACPCh. 23.5 - If drinking from a polycarbonate bottle, does a 15...Ch. 23.5 - Assume you weigh 156 lb. How much BPA do you...Ch. 23.5 - Prob. 3.5ACPCh. 23 - Prob. 1PSCh. 23 - Prob. 2PSCh. 23 - Is violet light (with a wavelength of 400 nm)...Ch. 23 - Prob. 4PSCh. 23 - Prob. 5PSCh. 23 - Prob. 6PSCh. 23 - Prob. 7PSCh. 23 - Prob. 8PSCh. 23 - Prob. 9PSCh. 23 - What is the molecular formula for an alkane with...Ch. 23 - Prob. 11PSCh. 23 - Prob. 12PSCh. 23 - One of the structural isomers with the formula...Ch. 23 - Prob. 14PSCh. 23 - Prob. 15PSCh. 23 - Give the systematic name for the following alkane....Ch. 23 - Draw the structure of each of the following...Ch. 23 - Draw structures for the following compounds. (a)...Ch. 23 - Prob. 19PSCh. 23 - Prob. 20PSCh. 23 - Draw the structure of the chair form of...Ch. 23 - Prob. 22PSCh. 23 - Prob. 23PSCh. 23 - Prob. 24PSCh. 23 - Prob. 25PSCh. 23 - Prob. 26PSCh. 23 - Prob. 27PSCh. 23 - What structural requirement is necessary for an...Ch. 23 - A hydrocarbon with the formula C5H10, can be...Ch. 23 - Prob. 30PSCh. 23 - Prob. 31PSCh. 23 - Prob. 32PSCh. 23 - The compound 2-bromobutane is a product of...Ch. 23 - The compound 2,3-dibromo-2-methylhexane is formed...Ch. 23 - Prob. 35PSCh. 23 - Prob. 36PSCh. 23 - Prob. 37PSCh. 23 - Prob. 38PSCh. 23 - Prob. 39PSCh. 23 - Give the systematic name for each of the following...Ch. 23 - Prob. 41PSCh. 23 - Write an equation for the preparation of...Ch. 23 - Prob. 43PSCh. 23 - Prob. 44PSCh. 23 - Prob. 45PSCh. 23 - Prob. 46PSCh. 23 - Prob. 47PSCh. 23 - Name the following amines: (a) CH3CH2CH2NH2 (b)...Ch. 23 - Draw structural formulas for the four possible...Ch. 23 - Prob. 50PSCh. 23 - Prob. 51PSCh. 23 - Prob. 52PSCh. 23 - Prob. 53PSCh. 23 - Prob. 54PSCh. 23 - Prob. 55PSCh. 23 - Prob. 56PSCh. 23 - Prob. 57PSCh. 23 - Prob. 58PSCh. 23 - Give the structural formula and systematic name...Ch. 23 - Prob. 60PSCh. 23 - Prob. 61PSCh. 23 - Prob. 62PSCh. 23 - Prob. 63PSCh. 23 - Prob. 64PSCh. 23 - Prob. 65PSCh. 23 - Prob. 66PSCh. 23 - Prob. 67PSCh. 23 - Prob. 68PSCh. 23 - Identify the functional groups in the following...Ch. 23 - Prob. 70PSCh. 23 - Prob. 71PSCh. 23 - Prob. 72PSCh. 23 - Prob. 73PSCh. 23 - Prob. 74PSCh. 23 - Prob. 75GQCh. 23 - Prob. 76GQCh. 23 - Prob. 77GQCh. 23 - Prob. 78GQCh. 23 - Prob. 79GQCh. 23 - Prob. 80GQCh. 23 - Prob. 81GQCh. 23 - Write equations for the following reactions,...Ch. 23 - Prob. 83GQCh. 23 - Prob. 84GQCh. 23 - Draw the structure of each of the following...Ch. 23 - Prob. 86GQCh. 23 - Prob. 87GQCh. 23 - Draw structural formulas for possible isomers with...Ch. 23 - Prob. 89GQCh. 23 - Prob. 90GQCh. 23 - Prob. 91GQCh. 23 - Prob. 92GQCh. 23 - Prob. 93GQCh. 23 - Prob. 94GQCh. 23 - Draw the structure of glyceryl trilaurate, a fat....Ch. 23 - Prob. 96GQCh. 23 - Prob. 97GQCh. 23 - Prob. 98GQCh. 23 - Prob. 99GQCh. 23 - There are three ethers with the formula C4H10O....Ch. 23 - Review the opening photograph about chocolate...Ch. 23 - Prob. 102GQCh. 23 - Prob. 103ILCh. 23 - Prob. 104ILCh. 23 - Prob. 105ILCh. 23 - Prob. 106ILCh. 23 - Prob. 107ILCh. 23 - Prob. 108ILCh. 23 - Prob. 109ILCh. 23 - Prob. 110ILCh. 23 - Prob. 111ILCh. 23 - Prob. 112ILCh. 23 - Prob. 113ILCh. 23 - Prob. 114ILCh. 23 - Prob. 115SCQCh. 23 - Prob. 116SCQCh. 23 - Prob. 117SCQCh. 23 - Prob. 118SCQCh. 23 - Prob. 119SCQCh. 23 - Prob. 120SCQCh. 23 - Prob. 121SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License