
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.4, Problem 23.7CYU
- (a) Name each of the following compounds and its functional group.
- (1) CH3CH2 CH2OH
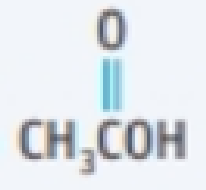
- (2) CH3CH2NH2
- (b) Name the product from the reaction of compounds 1 and 2 above.
- (c) What is the name and structure of the product from the oxidation of 1 with an excess of oxidizing agent?
- (d) Give the name and structure of the compound that results from combining 2 and 3.
- (e) What is the result of adding an acid (say HCl) to compound 3?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the structural formulas of compounds A, C, D, E and F and draw the isomer of B, with the explaination on which one would be the major product and why.
5. How many isomers are there Of C6H14?
6. Aside from a halogenated alkane, what other product results from the bromination of hexane? Write the formula
Draw the structures for and give the names for each of the isomeric carbonyl compounds corresponding to C4H8O and suggest suitable reactions for distinguishing between them
Chapter 23 Solutions
Chemistry & Chemical Reactivity
Ch. 23.2 - (a) Draw the nine isomers having the formula...Ch. 23.2 - Prob. 23.2CYUCh. 23.2 - There are 17 possible alkene isomers with the...Ch. 23.2 - Prob. 23.4CYUCh. 23.2 - Aniline, C6H5NH2, is the common name for...Ch. 23.3 - Draw the structure of 1-butanol and alcohols that...Ch. 23.4 - (a) Name each of the following compounds and its...Ch. 23.5 - Kevlar is a well-known polymer that is now used to...Ch. 23.5 - Prob. 1.1ACPCh. 23.5 - Prob. 1.2ACP
Ch. 23.5 - Prob. 1.3ACPCh. 23.5 - Prob. 2.1ACPCh. 23.5 - Prob. 2.2ACPCh. 23.5 - Prob. 2.3ACPCh. 23.5 - What is the atom economy for the reaction of...Ch. 23.5 - Prob. 3.2ACPCh. 23.5 - If drinking from a polycarbonate bottle, does a 15...Ch. 23.5 - Assume you weigh 156 lb. How much BPA do you...Ch. 23.5 - Prob. 3.5ACPCh. 23 - Prob. 1PSCh. 23 - Prob. 2PSCh. 23 - Is violet light (with a wavelength of 400 nm)...Ch. 23 - Prob. 4PSCh. 23 - Prob. 5PSCh. 23 - Prob. 6PSCh. 23 - Prob. 7PSCh. 23 - Prob. 8PSCh. 23 - Prob. 9PSCh. 23 - What is the molecular formula for an alkane with...Ch. 23 - Prob. 11PSCh. 23 - Prob. 12PSCh. 23 - One of the structural isomers with the formula...Ch. 23 - Prob. 14PSCh. 23 - Prob. 15PSCh. 23 - Give the systematic name for the following alkane....Ch. 23 - Draw the structure of each of the following...Ch. 23 - Draw structures for the following compounds. (a)...Ch. 23 - Prob. 19PSCh. 23 - Prob. 20PSCh. 23 - Draw the structure of the chair form of...Ch. 23 - Prob. 22PSCh. 23 - Prob. 23PSCh. 23 - Prob. 24PSCh. 23 - Prob. 25PSCh. 23 - Prob. 26PSCh. 23 - Prob. 27PSCh. 23 - What structural requirement is necessary for an...Ch. 23 - A hydrocarbon with the formula C5H10, can be...Ch. 23 - Prob. 30PSCh. 23 - Prob. 31PSCh. 23 - Prob. 32PSCh. 23 - The compound 2-bromobutane is a product of...Ch. 23 - The compound 2,3-dibromo-2-methylhexane is formed...Ch. 23 - Prob. 35PSCh. 23 - Prob. 36PSCh. 23 - Prob. 37PSCh. 23 - Prob. 38PSCh. 23 - Prob. 39PSCh. 23 - Give the systematic name for each of the following...Ch. 23 - Prob. 41PSCh. 23 - Write an equation for the preparation of...Ch. 23 - Prob. 43PSCh. 23 - Prob. 44PSCh. 23 - Prob. 45PSCh. 23 - Prob. 46PSCh. 23 - Prob. 47PSCh. 23 - Name the following amines: (a) CH3CH2CH2NH2 (b)...Ch. 23 - Draw structural formulas for the four possible...Ch. 23 - Prob. 50PSCh. 23 - Prob. 51PSCh. 23 - Prob. 52PSCh. 23 - Prob. 53PSCh. 23 - Prob. 54PSCh. 23 - Prob. 55PSCh. 23 - Prob. 56PSCh. 23 - Prob. 57PSCh. 23 - Prob. 58PSCh. 23 - Give the structural formula and systematic name...Ch. 23 - Prob. 60PSCh. 23 - Prob. 61PSCh. 23 - Prob. 62PSCh. 23 - Prob. 63PSCh. 23 - Prob. 64PSCh. 23 - Prob. 65PSCh. 23 - Prob. 66PSCh. 23 - Prob. 67PSCh. 23 - Prob. 68PSCh. 23 - Identify the functional groups in the following...Ch. 23 - Prob. 70PSCh. 23 - Prob. 71PSCh. 23 - Prob. 72PSCh. 23 - Prob. 73PSCh. 23 - Prob. 74PSCh. 23 - Prob. 75GQCh. 23 - Prob. 76GQCh. 23 - Prob. 77GQCh. 23 - Prob. 78GQCh. 23 - Prob. 79GQCh. 23 - Prob. 80GQCh. 23 - Prob. 81GQCh. 23 - Write equations for the following reactions,...Ch. 23 - Prob. 83GQCh. 23 - Prob. 84GQCh. 23 - Draw the structure of each of the following...Ch. 23 - Prob. 86GQCh. 23 - Prob. 87GQCh. 23 - Draw structural formulas for possible isomers with...Ch. 23 - Prob. 89GQCh. 23 - Prob. 90GQCh. 23 - Prob. 91GQCh. 23 - Prob. 92GQCh. 23 - Prob. 93GQCh. 23 - Prob. 94GQCh. 23 - Draw the structure of glyceryl trilaurate, a fat....Ch. 23 - Prob. 96GQCh. 23 - Prob. 97GQCh. 23 - Prob. 98GQCh. 23 - Prob. 99GQCh. 23 - There are three ethers with the formula C4H10O....Ch. 23 - Review the opening photograph about chocolate...Ch. 23 - Prob. 102GQCh. 23 - Prob. 103ILCh. 23 - Prob. 104ILCh. 23 - Prob. 105ILCh. 23 - Prob. 106ILCh. 23 - Prob. 107ILCh. 23 - Prob. 108ILCh. 23 - Prob. 109ILCh. 23 - Prob. 110ILCh. 23 - Prob. 111ILCh. 23 - Prob. 112ILCh. 23 - Prob. 113ILCh. 23 - Prob. 114ILCh. 23 - Prob. 115SCQCh. 23 - Prob. 116SCQCh. 23 - Prob. 117SCQCh. 23 - Prob. 118SCQCh. 23 - Prob. 119SCQCh. 23 - Prob. 120SCQCh. 23 - Prob. 121SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One mole of an unknown hydrocarbon, compound C, in the presence of a platinum catalyst, adds 98.9 L of hydrogen, measured at 744 mm Hg and 22 degrees C , to form a saturated alkane which contains one ring. When one mole of compound C is reacted with ozone, followed by reduction with (CH3)2S , four moles of only one product was formed, whose condensed molecular formula is CHO -CHO. Give the structure of compound C. Explain your reasoningarrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forwardA compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forward
- What reagent would you use to distinguish between the aldehydes and ketones and indicate what you would observearrow_forwardHello, please draw and name the organic molecules according to the following criteria (draw the entire structure out clearly with each C and H and other atoms and name it) a) The molecule must have a benzene, could potentially have a rxn with H2 using a platinum catalyst and has at least one branch b) The molecule must have an alcohol R-chain, could produce a carboxylic if reacted with KMn04 and has contains two or more branchesarrow_forwardGive 3 examples of combustion of alkanes and write its general reaction and mechanismarrow_forward
- 2)Write a balanced chemical equation for each of the following processes, Structural or displayed formulae shouldbe used for all organic substances,a)Making ethanol using ethene as feedstock. Include the formula of the catalyst used.b)The complete combustion of ethanol in oxygen.C)The dehydration of butan-2-ol when passed over hot AI2 O3. Give three equations, one for each of the three possible products,d)The reaction of ethanoic acid with ethanol. Name the catalyst used, the type of reaction andthe products.arrow_forward(a) One test for the presence of an alkene is to add a smallamount of bromine, which is a red-brown liquid, and lookfor the disappearance of the red-brown color. This test doesnot work for detecting the presence of an aromatic hydrocarbon.Explain. (b) Write a series of reactions leading topara-bromoethylbenzene, beginning with benzene andusing other reagents as needed. What isomeric side productsmight also be formed?arrow_forwardAcid-catalyzed hydration of 2-Methyl-1-butene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explain why, by showing the structures of theproducts.arrow_forward
- Write a condensed structural formula for a dihydroxy compound with the formula C3H8O2.arrow_forward1. There are five constitutional isomers with the molecular formula C6H14. When treated with chlorine at 300°C, isomer A gives a mixture of two monochlorination products. Under the same conditions, isomer B gives a mixture of five monochlorination products, isomer C gives four monochlorination products, and isomer D gives a mixture of three monochlorination products. From this information, draw the structure of isomer B. 2. Is it possible to prepare 1-chloro-2,2-dimethylpropane in high yield by halogenation of an alkane? _____no/yes How many monohalo isomers are possible upon radical halogenation of the parent alkane? _____ (Consider stereoisomers as well.) Please answer very soon will give rating surely Both questions answers neededarrow_forwardDetermine the DOU for the following molecules and suggest a structure for each. C5H7Br2ONarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License