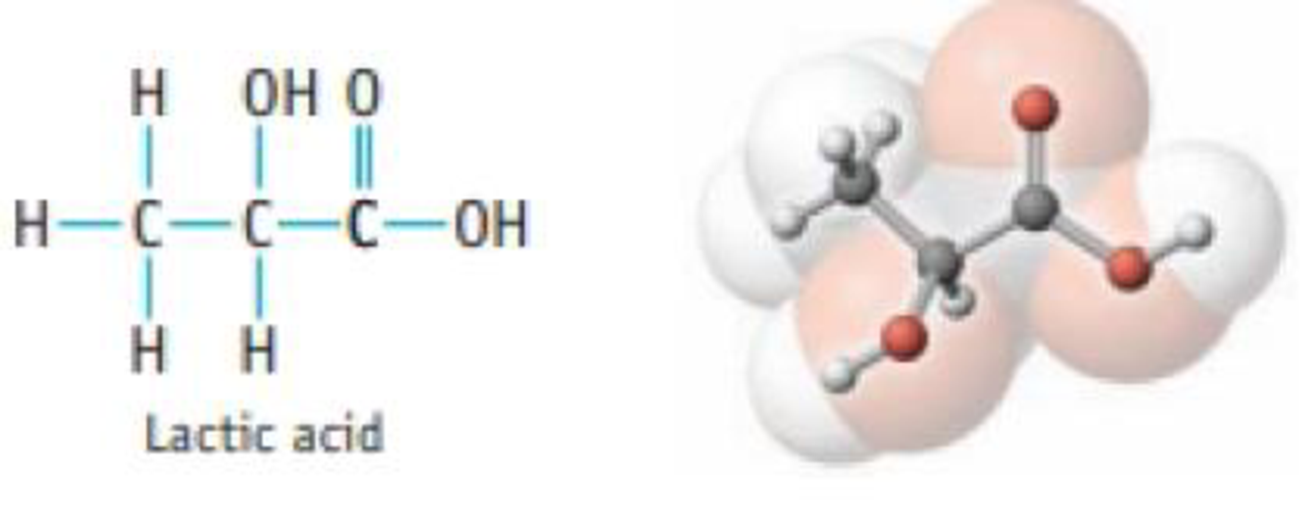
Problem 1PS: The equation for the oxidation of phosphorus in air is P4(s) + 5 O2(g) P4O10(s). Identify the... Problem 2PS: Write an equation from the following description: reactants are gaseous NH3 and O2, products are... Problem 3PS: The equation for the reaction of phosphorus and chlorine is P4(s) + 6 Cl2(g) 4 PCl3(). If you use... Problem 4PS: The equation for the reaction of aluminum and bromine is 2 Al(s) + 3 Br2() Al2Br6(s). If you use... Problem 5PS: Oxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO2. How many grams... Problem 6PS: A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of... Problem 7PS: Write balanced chemical equations for the following reactions. (a) The reaction of aluminum and... Problem 8PS: Write balanced chemical equations for the following reactions: (a) production of ammonia, NH3(g), by... Problem 9PS Problem 10PS Problem 11PS Problem 12PS: Balance the following equations, and name each reactant and product: (a) SF4(g) + H2O() SO2(g) +... Problem 13PS Problem 14PS Problem 15PS: Equal amounts of two acidsHCl and HCO2H (formic acid)are placed in aqueous solution. When... Problem 16PS Problem 17PS: What is an electrolyte? How can you differentiate experimentally between a weak electrolyte and a... Problem 18PS: Name and give the formulas of two acids that are strong electrolytes and one acid that is a weak... Problem 19PS: Which compound or compounds in each of the following groups is (are) soluble in water? (a) CuO,... Problem 20PS: Which compound or compounds in each of the following groups is (are) soluble in water? (a) BaSO4,... Problem 21PS: The following compounds are water-soluble. What ions are produced by each compound in aqueous... Problem 22PS: The following compounds are water-soluble. What ions are produced by each compound in aqueous... Problem 23PS: Decide whether each of the following is water-soluble. If soluble, tell what ions are produced when... Problem 24PS: Decide whether each of the following is water-soluble. If soluble, tell what ions are produced when... Problem 25PS: Balance the equation for the following precipitation reaction, and then write the net ionic... Problem 26PS: Balance the equation for the following precipitation reaction, and then write the net ionic... Problem 27PS: Predict the products of each precipitation reaction. Balance the equation, and then write the net... Problem 28PS Problem 29PS: Write a balanced equation for the ionization of nitric acid in water. Problem 30PS: Write a balanced equation for the ionization of perchloric acid in water. Problem 31PS Problem 32PS: Phosphoric add can supply one, two, or three H3O+ ions in aqueous solution. Write balanced equations... Problem 33PS Problem 34PS Problem 35PS Problem 36PS Problem 37PS Problem 38PS Problem 39PS: Write an equation that describes the equilibrium that exists when nitric acid dissolves in water.... Problem 40PS: Write an equation that describes the equilibrium that exists when the weak acid benzoic acid... Problem 41PS Problem 42PS: Write two chemical equations, one in which H2PO4 is a Brnsted acid (in reaction with the carbonate... Problem 43PS: Balance the following equations, and then write the net ionic equation. (a) (NH4)2CO3(aq) +... Problem 44PS: Balance the following equations, and then write the net ionic equation: (a) Zn(s) + HCl(aq) H2(g) +... Problem 45PS Problem 46PS: Balance each of the following equations, and then write the net ionic equation. Show states for all... Problem 47PS: Write balanced net ionic equations for the following reactions: (a) the reaction of nitrous add (a... Problem 48PS: Write balanced net ionic equations for the following reactions: (a) the reaction of aqueous... Problem 49PS: Siderite is a mineral consisting largely of iron(II) carbonate. Write an overall, balanced equation... Problem 50PS: The mineral rhodothrosite is manganese() carbonate. Write an overall, balanced equation for the... Problem 51PS Problem 52PS Problem 53PS: Determine the oxidation number of each element in the following ions or compounds. (a) BrO3- (b)... Problem 54PS: Determine the oxidation number of each element in the following ions or compounds. (a) PF6 (b)... Problem 55PS Problem 56PS: Which two of the following reactions are oxidation-reduction reactions? Explain your answer briefly.... Problem 57PS: In the following reactions, decide which reactant is oxidized and which is reduced. Designate the... Problem 58PS: In the following reactions, decide which reactant is oxidized and which is reduced. Designate the... Problem 59PS: Balance the following equations, and then classify each as a precipitation, acid-base, or... Problem 60PS Problem 61PS: Classify each of the following reactions as a precipitation, acid-base, or gas forming reaction.... Problem 62PS Problem 63PS: Balance each of the following equations, and classify them as precipitation, acid-base, gas-forming,... Problem 64PS: Complete and balance the equations below, and classify them as precipitation, acid-base,... Problem 65PS Problem 66PS Problem 67GQ: Balance the following equations: (a) for the synthesis of urea, a common fertilizer CO2(g) + NH3(g) ... Problem 68GQ: Balance the following equations: (a) for the reaction to produce "superphosphate" fertilizer... Problem 69GQ Problem 70GQ: Give the formula for each of the following compounds: (a) a soluble compound containing the acetate... Problem 71GQ Problem 72GQ: Name two anions that combine with Al3+ ion to produce water-soluble compounds. Problem 73GQ: Write the net ionic equation and identify the spectator ion or ions in the reaction of nitric acid... Problem 74GQ: Identify and name the water-insoluble product in each reaction and write the net ionic equation: (a)... Problem 75GQ: Bromine is obtained from sea water by the following redox reaction: Cl2(g) + 2 NaBr(aq) 2 NaCl(aq)... Problem 76GQ: Identify each of the blowing substances as a likely oxidizing or reducing agent: HNO3, Na, C12, O2,... Problem 77GQ: The mineral dolomite contains magnesium carbon-ate. This reacts with hydrochloric add. MgCO3(s) + 2... Problem 78GQ: Aqueous solutions of ammonium sulfide, (NH4)2S, and Hg(NO3)2 react to produce HgS and NH4NO3. (a)... Problem 79GQ Problem 80GQ Problem 81GQ: Balance equations for these reactions that occur in aqueous solution, and then classify each as a... Problem 82GQ Problem 83GQ: You are given mixtures containing the following compounds. Which compound in each pair could be... Problem 84GQ: Identify, from each list below, the compound or compounds that will dissolve in water to give a... Problem 85GQ Problem 86GQ Problem 87GQ: Gas evolution was observed when a solution of Na2S was treated with acid. The gas was bubbled into a... Problem 89IL Problem 90IL Problem 91IL Problem 92IL: A Suggest a laboratory method for preparing barium phosphate. (See Study Question 97 for a way to... Problem 93IL: The Toliens test for the presence of reducing sugars (say, in a urine sample) involves treating the... Problem 94SCQ: There are many ionic compounds that dissolve in water to a very small extent. One example is... Problem 95SCQ: Most naturally occurring acids are weak acids. Lactic acid is one example.... Problem 96SCQ: You want to prepare barium chloride, BaC12, using an exchange reaction of some type. To do so, you... Problem 97SCQ Problem 98SCQ: A Describe how to prepare zinc chloride by (a) an add-base reaction, (b) a gas-forming reaction, and... Problem 99SCQ: A common method for analyzing for the nickel content of a sample is to use a precipitation reaction.... Problem 101SCQ: The presence of arsenic in a sample that may also contain another Group 5A element, antimony, can be... format_list_bulleted


 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
