
Concept explainers
(a)
Interpretation:The natural
Concept introduction:
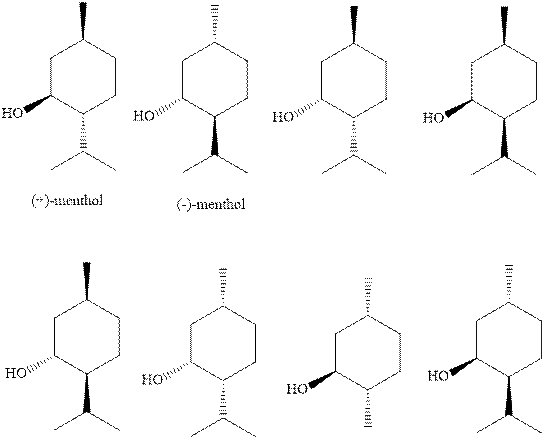
(b)
Interpretation: The natural
Concept introduction:
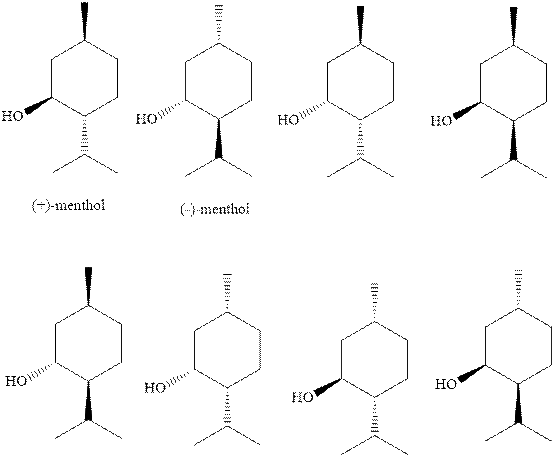
(c)
Interpretation: The natural
Concept introduction:
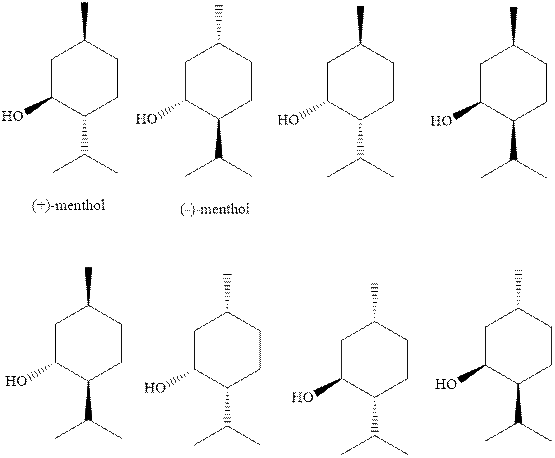
(c)
Interpretation: The stability order for the three diastereomers namely menthol, isomenthol, and neomenthol should be determined.
Concept introduction:In chair form all the
bond further allows for no amount of torsional strain. There are two kinds of hydrogens in cyclohexane namely axial and equatorial indicated as follows:
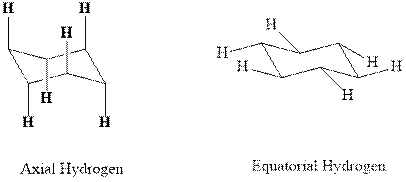
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Rank the following groups in order of decreasing priority. −F, −NH2, −CH3, −OHarrow_forwardDraw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.)E,Z isomerism but not chiralityarrow_forwardIf I chlorinate 2-bromo-1-chloropropane under light, how many products do I obtain? Are any chiral?arrow_forward
- Compound X, C8H17Cl, is a chiral product of the radical chlorination of 4-methylheptane.X reacts in SN2 fashion with NaI in acetone to form Z, C8H17I. When the reactant is the R-enantiomer of X, only the R-enantiomer of Z is formed.Draw a structural formula for X; do not show stereochemistry.arrow_forwardDraw and name each of the following: a. An ether with molecular formula C3H8O b. An alkene with the formula C5H10 that has stereoisomers. c. A tertiary (30) alcohol with molecular formula C5H11OH that is chiral. d. A primary (10) aliphatic amine with molecular formula C3H9 e. A thiol with molecular formula C6H14arrow_forwardDraw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forward
- Draw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.) Neither E,Z isomerism nor chiralityarrow_forwardIgnoring stereoisomers, draw the two possible enols for butan-2-one (CH3COCH2CH3), and predict which one is more stable.arrow_forward1,4-Pentadiene (CH2=CH-CH2-CH=CH2) is a liquid at room temperature and has a density of 0.66 g/mL and molar mass of 68.12 g/mol. In a laboratory experiment, 3.80 mL of this compound was treated with 4.80 mL of conc. H2SO4 (100% w/w; molar mass 98.08 g/mol). Note that the density of conc. H2SO4 is 1.84 g/mL. The resulting sulfate ester was then treated with 1.20 mL of water (molar mass 18.02 g/mol) affording, after work- up, 2,4-pentanediol (molar mass 104.15 g/mol) as the crude product. The crude product was then purified by simple distillation, which yielded 2.00 g of pure product. a. Provide a balanced chemical equation to show the reaction between 1,4-pentadiene and sulfuric acid. Do not use molecular formulas in the chemical equation except for sulfuric acid. b. What reactant is the limiting reagent in this chemical equation? Show calculations to support your answer.arrow_forward
- 1. Name and draw all possible isomers of trimethylcyclopropane. Which of the isomers is chiral?2. If this is chiral, then is it an R or S isomer?arrow_forwarda: Is the product chiral? _____ b: Assuming that the reaction takes place with equal likelihood from both Re and Si faces of the carbonyl group, is the product optically active? ____arrow_forwardPlease complete the following related questions i) Draw all possible isomers for the chemical formula Ma3bcd.How many are there in total? How many pairs of enantiomers are there?ii) Repeat for formula Ma3b2c.arrow_forward
