
Concept explainers
Interpretation: Each stereocenter in the indicated structures should be labeled as either R or S.
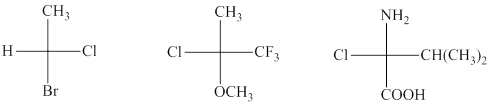
Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- R and S Assignments in Compounds with Two or More Stereogenic Centers ? Define this ?arrow_forwardAs a fischer projection is the compound R or S and which group is the highest priority ?arrow_forwardMake sure to answer the quesi to chem below Using the priority rule, identify the configuration at each chiral center and the configuration of the double bond: For a chiral center please type EXACTLY one of the following: R or S or neither if it is not a chiral center. For a double bond please type EXACTLY one of the following: E or Z or neither if the double bond has no stereochemistry.arrow_forward
- Which term best describes the following rxn? A. regioselective B. stereoselective C. both D. neitherarrow_forwardDraw all possible configurational stereoisomers for the given molecule below. Designate the R/S on the stereogenic centers and E/Z on the C=Carrow_forwardAssign R and S, is this an enantiomer, diastereomer, constitutional isomer, or identical?arrow_forward
- Label stereogenic centers and determined R\S configuration. Then label products of reactions as either chiral or achiralarrow_forwardIdentify the relationship between the following pairs of structures, then state whether they are enantiomers, diastereomers or identical. Help me answer iv., v., iv., vii. (last 4 sub part)arrow_forwarda) Draw a 2-dimensional structure of 4,5-dibromooctane and label, with an asterisk, thetetrahedral stereo centers.b) What is the maximum number of stereoisomers for this molecule?c) Draw Fischer projections of all the potential stereoisomers of (a).d) Label, on the above projections, all the stereocenters as R or S.e) Indicate the enantiomers, diastereomers and the meso compound if present.arrow_forward
- For each of the following compounds, give the correct R/S notation for all stereocentersarrow_forward(A) How many chirality centers does the following molecule contain? (B) How many stereoisomers are possible for this molecule? (C) Assign R,S designation to each chiral carbonarrow_forwardIn a fischer projection, is the molecule r or s, and what is the highest priority?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
