
(a)
Interpretation:The natural products indicated should be identified as either chiral or achiral.
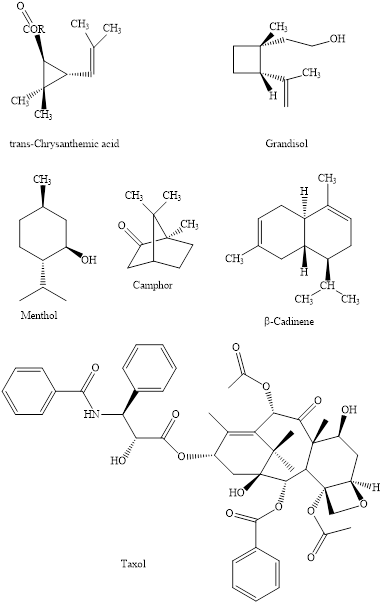
Concept introduction:
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Four kinds of symmetry elements that may be present are tabulated as follows:
Presence of any symmetry element makes molecule achiral and optically inactive.
(b)
Interpretation: The three molecules that represent enantiomers of
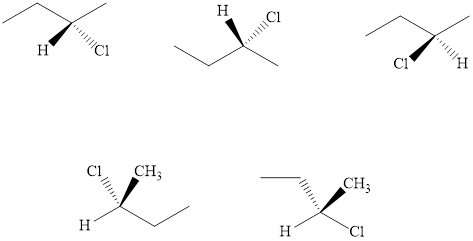
Concept introduction: The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic Chemistry: Structure and Function
- write structural formulas for all the compounds that are trichloro derivatives of cyclopropane, which are chiral and which are achiral?arrow_forwardDraw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.)E,Z isomerism but not chiralityarrow_forward(S)-2-Tosylpentane (stereochemistry not shown in the figure below) is converted to one of the stereoisomers of 2-bromopentane. Provide the formula of the reagent you would use to accomplish this transformation, and draw the correct stereoisomer product.arrow_forward
- Explain briefly and clearly the following concepts, taking as reference the molecule of n-butane and the corresponding drawings or illustrations. See pages 149-152 of the book Organic Chemistry, sixth edition (J. G. Smith). 4. What is steric hindrance in a conformation? Then draw a picture to illustrate the concept? 5. What is the torsional stress of a conformation? Then draw a picture to illustrate the concept? 6. Describe 1,3-diaxial interaction and illustrate with a specific example.arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Which of these is the lowest energy conformation?arrow_forwardDoes a unit of unsaturation of 5 signify anything?arrow_forward
- 1, part A) We observe 6 carbon stereocenters in the molecule below. Indicate each stereocenter, and give the absolute S or R configuration. 1, part B) Indicate each stereoisomer in this molecule, and give the absolute S or R configuration. Then, indicate E or Z configuration of all alkenes in it (*ignoring aromatic ring*).arrow_forwardA difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired conguration.a. Label this stereogenic center as R or S.b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship.c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forwardA) Considering compounds 2a through 2l, identify: 1)one pair of geometric isomers 2)two pairs of enantiomers and 3)three pairs of identical molecules B) Give the names, including the configurations, of each of the geometric isomers and of each of the enantiomers identified in 1A and 1B. Draw the relevant structures. C) Sort compounds 2a, 2b, 2c, 2f and 2k in order of increasing solubility in water and briefly justify.arrow_forward
- Give the most stable conformation for (1S, 2S)-1-methoxy-2-chlorocyclohexane.arrow_forwardWrite a conformational structure for 1,2,3-trimethylcyclohexane in which all the methyl groups are axial and then show its more stable conformation.arrow_forwardgive the degree of unsaturation of the compounds: Vanillin(C8H8O3) and ethyl ethocyacetate (C6H12O3). and draw their structuresarrow_forward
