
a)
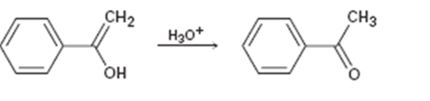
Interpretation:
Given that the final step in the hydration of an
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation ion then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
b)
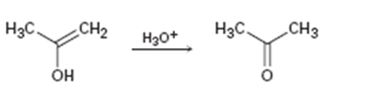
Interpretation:
Given that the final step in the hydration of an alkyne under acidic conditions is the tautomerization of the enol intermediate to give the corresponding ketone. The mechanism involves protonation followed by deprotonation. Based on this information a mechanism for the tautomerization reaction shown is to be drawn.
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
c)
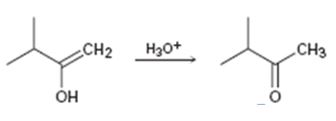
Interpretation:
Given that the final step in the hydration of an alkyne under acidic conditions is the tautomerization of the enol intermediate to give the corresponding ketone. The mechanism involves protonation followed by deprotonation. Based on this information a mechanism for the tautomerization reaction shown is to be drawn.
Concept introduction:
The enol picks up a proton from H3O+ using the unshared pair of electrons on oxygen and the π electrons in the double bond to yield a carbocation. The carbocation then loses a proton and the electrons in the O-H bond are moved towards the positively charged carbon to yield the ketone.
To propose:
A mechanism for the tautomerization reaction shown.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- Predict the elimination and substitution products of the following reactionarrow_forwardWhat is the major organic product obtained form the following sequence of reactions (assume that mixtures of ortho and para disubstituted compounds can be separated; continue the synthesis with either one)?arrow_forwardComplete the following multistep syntheses by showing the major products and the reagents nessesary for each steparrow_forward
- Provide the major product including stereochemistry for the following reaction.arrow_forwardProvide the major product and indicate which mechanismarrow_forwardThe reaction of the following cyclic ester with excess methyl magnesium bromide and water with the mechanism and name the resulting product.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

