
a)
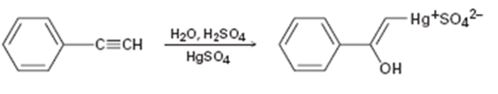
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of phenylacetylene is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of ethynylbenzene.
b)
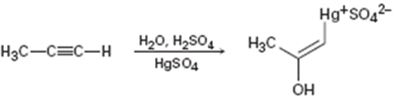
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of propyne is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of propyne.
c)
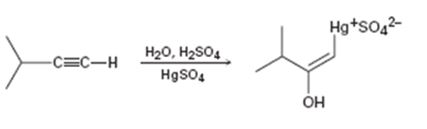
Interpretation:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of 3-methyl-1-butyne is to be drawn.
Concept introduction:
In the first step attack of the π electrons of the triple bond on the electrophilic Hg2+ ion takes place to yield a mercury containing vinylic carbocation intermediate. In the second step nucleophilic attack of water takes place on the carbocation. A new C-O bond is formed leading to the formation of a protonated mercury containing enol. In the third step water abstracts a proton from the protonated enol to yield the organomercury intermediate.
To draw:
The electron pushing mechanism for the formation of the organo-mercury intermediate obtained during the mercury catalyzed hydration of 3-methyl-1-butyne.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardUnder normal mild conditions, ketones are quite resistant to oxidation. But when treated with peracids (such as peracitic acid), they undergo an oxidation reaction. Explain how this happens using the following reaction showing a mechanism for the reaction. Cyclopentanone + peracetic acidarrow_forward
- As we will learn in Chapter 9, an epoxide is an ether with an oxygen atom in a three-membered ring. Epoxides can be made by intramolecular SN2 reactions of intermediates that contain a nucleophile and a leaving group on adjacent carbons, as shown.Assume that each of the following starting materials can be converted to an epoxide by this reaction. Draw the product formed (including stereochemistry) from each starting material. Why might some of these reactions be more difficult than others in yielding nucleophilic substitution products?arrow_forwardWhich of the following reaction yields a carbonyl-containing product? a Ozonolysis b Mild oxidation c Epoxidation d All of the abovearrow_forwardWhat is the major substitution product for the following reaction? Show the mechanism for the reaction.arrow_forward
- Draw the ALL of the E2 organic product formed when the structure shown below undergoes dehydrohalogenation in alcoholic KOH with heat. Which of the products is formed the most?arrow_forwardWhich of the following is FALSE regarding terminal alkynes? The proton in the terminal carbon is weakly acidic. They react with H₂O, H₂SO4, HgSO4 to produce an aldehyde. The hydrohalogenation reaction yields a geminal dihalide. Treatment with alcoholic AgNO3 produces a silver acetylide.arrow_forwardIdentify the reagents for each of the following reactions:arrow_forward
- Non-conjugated β,γ-unsaturated ketones, such as 3-cyclohexenone, as in acid-catalysed equilibrium with their α,β-unsaturated isomers. The mechanism has several intermediates. Draw the structure of the second reaction intermediate in the conversion of 3-cyclohexenone to 2-cyclohexenone. This intermediate is a neutral compound.arrow_forwardProvide reaction mechanisms for the following transformationsarrow_forwardWhat product is obtained when the following compound undergoes two successive elimination reactions?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


