
Concept explainers
a)
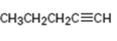
Interpretation:
How to synthesize 1-pentyne from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal
To state:
How to synthesize 1-pentyne from acetylene using any alkyl halide with four or fewer number of carbons.
b)
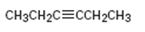
Interpretation:
How to synthesize 3-hexyne from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. Acetylene has two acidic hydrogens. Both hydrogens can be replaced by alkyl groups through this process.
To state:
How to synthesize 3-hexyne from acetylene using any alkyl halide with four or fewer number of carbons.
c)
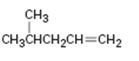
Interpretation:
How to synthesize 4-methyl-1-pentene from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. The alkyne can be reduced to the corresponding
To state:
How to synthesize 4-methyl-1-pentene from acetylene using any alkyl halide with four or fewer number of carbons.
d)
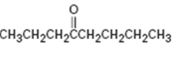
Interpretation:
How to synthesize 4-octanone from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. The alkyne undergoes hydration when treated with dilute H3SO4 in the presence of HgSO4 to yield an enol which tautomerizes to a
To state:
How to synthesize 4-octanone from acetylene using any alkyl halide with four or fewer number of carbons.
e)
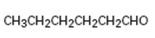
Interpretation:
How to synthesize hexanal from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkynes needed. The alkynes yield enols with OH on terminal carbon in hydroboration-oxidation reaction which tautomerize to yield
To state:
How to synthesize hexanal from acetylene using any alkyl halide with four or fewer number of carbons.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





