
a)
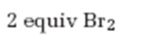
Interpretation:
The product formed when 2-hexyne reacts with 2 equivalents of bromine.
Concept introduction:
In addition reactions,
To give:
The product formed when 2-hexyne reacts with two molar equivalents of bromine.
b)
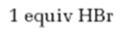
Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with two molar equivalents of a reagent yield the derivatives of the corresponding alkane as the product. With one molar equivalent of a reagent they yield a derivative of the corresponding alkene as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with 1 equivalent of HBr.
c)

Interpretation:
The product formed when 2-hexyne reacts with 1 equivalent of HBr is to be predicted.
Concept introduction:
In addition reactions, alkynes when treated with excess amount of a reagent yield the derivatives of the corresponding alkane as the product. The addition to unsymmetrical alkenes follows Markovnikov regiochemistry. The negative part of the reagent adds on to the more highly substituted carbon in triple bond.
To predict:
The product formed when 2-hexyne reacts with excess of HBr.
d)
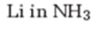
Interpretation:
The product formed when 2-hexyne reacts with Li in ammonia.
Concept introduction:
Alkynes can be reduced by treatment with hydrogen in the presence of a catalyst. The hydrogenation occurs with anti stereochemistry to give a trans- alkene as product if Li in ammonia is used.
To predict:
The product formed when 2-hexyne reacts with Li in ammonia.
e)
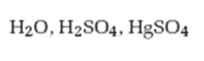
Interpretation:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4 is to be given.
Concept introduction:
When treated with H2O, H2SO4 in the presence of HgSO4 alkynes get hydrated to produce enols. The addition of water to unsymmetrical alkynes follows Markonikov regiochemistry while it cannot be applied in the case of symmetrical alkynes. The enols produced then undergo tautomerization to give a
To predict:
The product formed when 2-hexyne reacts with H2O, H2SO4 in the presence of HgSO4.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardWhen cis-2-decalone is dissolved in ether containing a trace of HCl, an equilibrium is established with trans-2-decalone. The latter ketone predominates in the equilibrium mixture. Propose a mechanism for this isomerization and account for the fact that the trans isomer predominates at equilibrium.arrow_forward
- Tranexamic acid, a drug useful against blood clotting, is prepared commercially from p-methylbenzonitrile. Formulate the steps likely to be used in the synthesis. (Don’t worry about cis-trans isomers; heating to 300 C interconverts the isomers.)arrow_forwardTreatment of p-bromotoluene with NaOH at 300°C yields a mixture of two products, but treatment of m-bromotoluene with NaOH yields a mixture of three products. Explain.arrow_forwardBenzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3. a. What are the major and minor products of the following reaction? b. Would you expect epoxides to undergo similar reactions?arrow_forward
- 8.b)Provide mechanisms for the following reactions:arrow_forward4 Part 3. Give the IUPAC names of the following compoundsPart 5: Predict the products, if any, of the following reactions.arrow_forwardReaction of 2-methyl-2-pentene with reagent is regioselective. Draw a structural formula for the product of reaction and account for the observed regioselectivity. Q.) Hg(OAc)2 in H2Oarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

