
a)
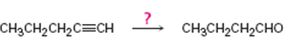
Interpretation:
How to synthesize butanal from 1-pentyne is to be shown.
Concept introduction:
To show:
How to synthesize butanal from 1-pentyne.
b)
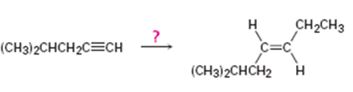
Interpretation:
How to synthesize trans- 6-methyl-3 heptene from 4-methyl-1-pentyne is to be shown.
Concept introduction:
Higher alkynes can be prepared first by converting the lower alkyne in to an alkynide by reacting with NaNH2 in liquid NH3 followed by the reaction of the alkynide with
To show:
How to synthesize trans- 6-methyl-3 heptene from 4-methyl-1-pentyne.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
