
Concept explainers
(a)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound should be determined.
Concept Introduction:
Valence electrons: The outermost electrons present in the compound other than the inner core electrons are denoted as valence electrons. The valence electrons are responsible for the bond formation.
There are about 19 valence electrons present in the given compound.
(a)
Explanation of Solution
The given compound
Similarly the chlorine atom contains 7 valence electrons in its outer most shell which totally comprises of
(b)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound
Concept introduction:
Lewis dot structure: It is the representation of chemical substance that how the valence are represented as dot and placed around the each atom present in the substance by considering the number of atoms with their valence electrons and number of bonds present in the given chemical substance.
(b)
Answer to Problem 116SCQ
The electron dot structure for the given compound is as follows,
Explanation of Solution
The electron dot structure for the given
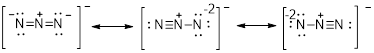
There are two oxygen atoms and one chlorine atom present in the given compound.
The oxygen atom contains 8 electrons that is represented as follows,
Similarly, the electrons present in the chlorine is represented as follows
Therefore, the chemical substance totally, has 20 valence electrons with it since addition of valence electrons present in the atom of the given compound says that. There are 2 O atoms each with 6 valence electrons one chlorine atom with 7 valence electrons and one negative charge which denotes present of one extra electrons which totally equals to 20.
The valence electrons are represented as dot around each atom present in the compound such that each atom tends to exhibit stable configuration that is 8electrons in its valence shell.
(c)
Interpretation:
For the given reaction the under given conditions the angle and the mass of
Concept introduction:
Hybridization refers to mixing of orbitals. The orbitals of different atoms overlap with each other and form a set of new orbitals called hybrid orbitals. The number of orbitals that are combined equal to the number of hybrid orbitals formed by combination.
An isolated atom remains in excited state and comes back to ground state. In this situation, it is difficult for orbitals to hybridize.
Hybridization is a hypothetical concept. It refers to mixing of atomic orbitals and the resultant orbitals formed are known as hybrid orbitals. After hybridization, the orbitals cannot be distinguished individually. Based on hybridization one can predict the geometry of the molecule though some deviations do exist.
(c)
Answer to Problem 116SCQ
The chlorine atom in the given compound is found to be
Explanation of Solution
The given compound contains chlorine with two oxygen atoms results to have two bonds with it.
The electronic configuration of Chlorine and oxygen is as follows,
Electronic configuration of chlorine is
The available orbitals that is s orbitals, one p orbital from chlorine and two p orbitals from oxygen atom gets hybridized and results to form new hybrid orbitals classified as
The central chlorine in compound
(d)
Interpretation:
For the given reaction the under given conditions the valence electrons present in the given compound
Concept introduction:
Bond angle: The angle formed between two bonds that is where two atoms gets bonded with the third central atom present.
(d)
Answer to Problem 116SCQ
The species
Explanation of Solution
The central chlorine in compound
Therefore,
(e)
Interpretation:
For the given reaction the under given conditions the mass of
Concept introduction:
Ideal gas equation:
Any gas is described by using four terms namely pressure, volume, temperature and the amount of gas. Thus combining three laws namely Boyle’s, Charles’s Law and Avogadro’s Hypothesis the following equation could be obtained. It is referred as ideal gas equation.
Under some conditions gases don not behave like ideal gas that is they deviate from their ideal gas properties. At lower temperature and at high pressures the gas tends to deviate and behave like real gases.
Boyle’s Law:
At given constant temperature conditions the mass of given ideal gas in inversely proportional to its volume.
Charles’s Law:
At given constant pressure conditions the volume of ideal gas is directly proportional to the absolute temperature.
Avogadro’s Hypothesis:
Two equal volumes of gases with same temperature and pressure conditions tend to have same number of molecules with it.
The relationship between partial pressure and
(e)
Answer to Problem 116SCQ
The mass of given compound formed is equal to
Explanation of Solution
Given:
First the given mass of
There are about
Then, the amount of
Now, analyzing the given chemical equation it is evident that 2 moles of
Similarly, there are
Analyzing the above calculations we have only about
Examining the given chemical reaction show that 1 mole of
Therefore, available amount of
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry & Chemical Reactivity
- Imagine that you have 4 buckets full of dark, muddy water. One bucket is frozen, one bucket is boiled, one bucket is shaken up, and one bucket is passed thru a paper filter. Which bucket is most likely to become lighter in color?arrow_forward(a) When chlorine atoms react with atmospheric ozone,what are the products of the reaction? (b) Based on averagebond enthalpies, would you expect a photon capable ofdissociating a C—Cl bond to have sufficient energy to dissociatea C—Br bond? (c) Would you expect the substanceCFBr3 to accelerate depletion of the ozone layer?arrow_forward2??arrow_forward
- What is the oxydation number of sulfur in sulfite ion, SO3(2-)?arrow_forwardCalculate the electrostatic part of the lattice energy of sodium chloride, given that the internuclear separation between Na1 and Cl2 ions is 2.82 Å.arrow_forwardDescribe in details the twelve-basic principle of Green Chemistry.arrow_forward
- What is the energy change when the temperature of 11.3 grams of gaseous krypton is decreased from 37.4 °C to 20.5 °C ?arrow_forwardHow should the mercury bulb of the thermometer be placed in the distillation apparatus? Why is the placement of the thermometer is important during distillation?arrow_forwardPotassium iodide (KI) exhibits predominantly ionic bonding. The K+ and I− ions have electron structures that are identical to which two inert gases?arrow_forward
- What mass of sodium hydroxide ,NaOH, would be required to produce 5.97 g of the antacid milk of magnesia,Mg(OH)2,by the reaction of MgCl2with NaOHarrow_forwardWhat is the result of like charges, be they phosphates or magnets, being in close proximity?arrow_forwardThe student finds the following particulate model of AgCl(s). Assuming the crystal structures are similar, how should the student modify the model to represent CuCl(s)? Justify your answer in terms of the radius and arrangement of electrons in the ions.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


