
Concept explainers
(a)
Interpretation:Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
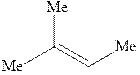
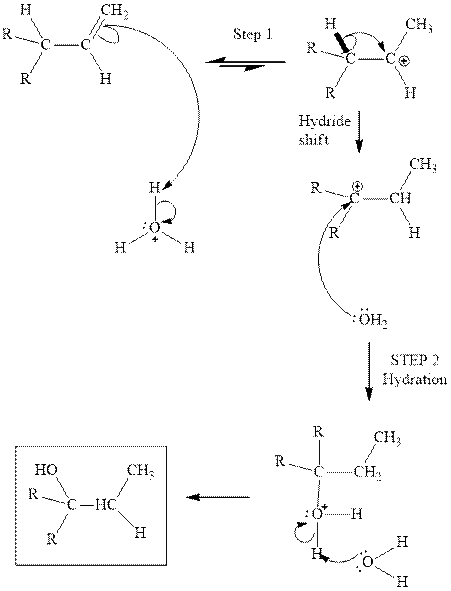
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
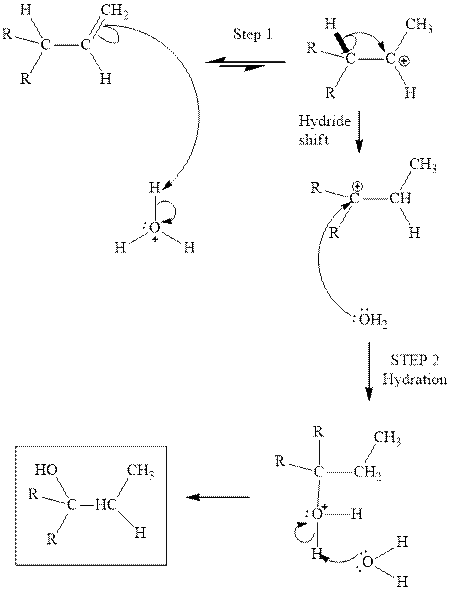
(b)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
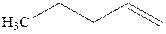
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
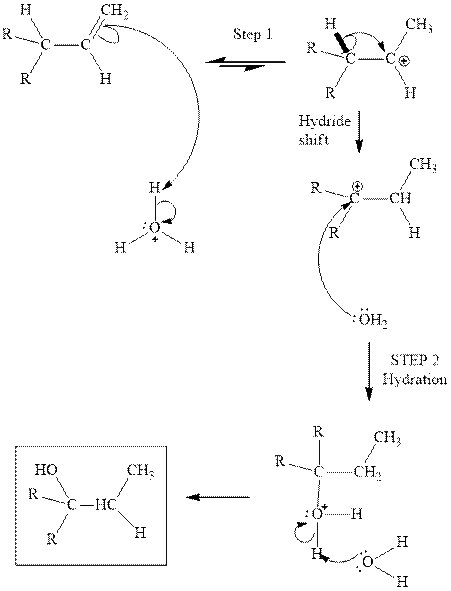
(c)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
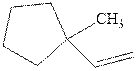
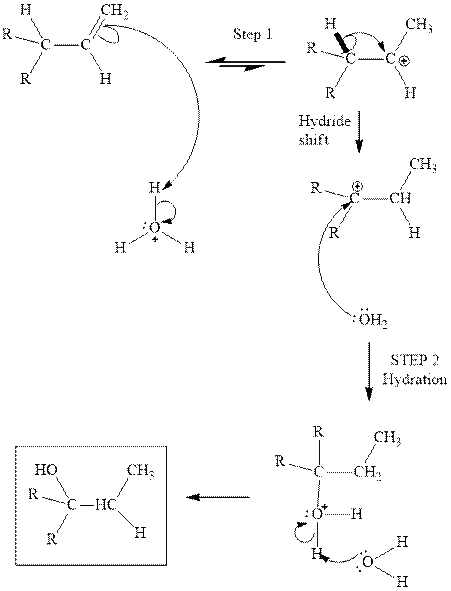
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
in the first stage. In the second stage, water itself acts as a nucleophile and another water abstracts a proton to give final hydration product as illustrated below.
(d)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
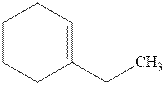
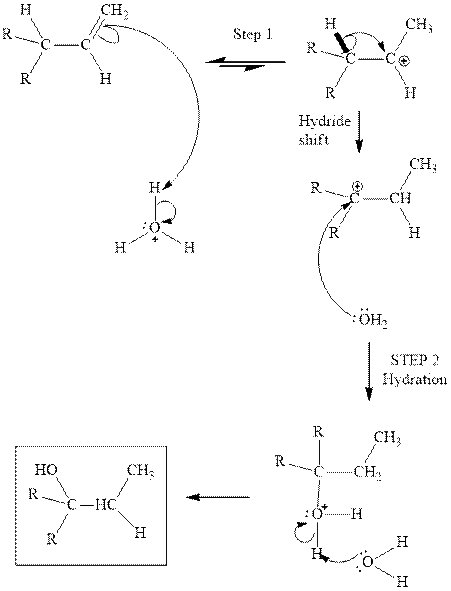
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
(e)
Interpretation: Structure of the alcohol that would be the major product of acid-catalyzed hydration should be written.
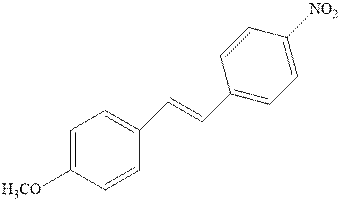
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst are
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Begin by classifying the carbon atoms on either side of the oxygen atom. Propose the types of alcohol and halide which can be used for Williamson ether synthesis.arrow_forwardDiscuss and explain in detail what phenols are, its properties, the different tests used to identify them and compare phenols to alcohols, its properties and lucas test conducted to compare the reactivity of primary, secondary and tertiary alcohols.arrow_forward1-pentanol + NaBr/H2SO4 -> 1-bromopentane Is this substitution reaction reversible? In other words, would one expect to obtain an equilibrium mixture of the alcohol and bromoalkene at the end of the reaction of would one expect this reaction to go to completion? Justify your answer?arrow_forward
- 1. In the reactions involving the three isomeric alcohols with the formula C4H9OH, describewhat each of the following tests showed about reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols.• the test with neutral KMnO4• the test with concentrated HCl2. Predict how the fourth alcohol with the formula C4H10O would react if tested with:• 0.01 M KMnO4• concentrated HCl at room temperatureExtend FurtherUse your observations of the solutions formed in the previous experiments and yourunderstanding of alcohols to complete a table like the one shown below. Research the meltingand boiling points to verify your answers.arrow_forwardAlkene can be prepared from alcohol using acid catalyzed dehydration reaction a. Draw and label the main aparatus and general important reagents that involved in the distillation process for this reaction. b. Suggest three (3) laboratory safety rules while conducting the reaction in 2(a) c. The prepared alkene reacts with bromine water with the presence of concentrated sulphuric acid. Suggest the reaction and explain your answer.arrow_forward11. Specify the appropriate alkene for the synthesis of the given alcohol by hydroboration-oxidation?arrow_forward
- Oxidation of a primary alcohol with pyridinium chlorochromate ( PCC, C5H6NCrO3Cl) would yield what?arrow_forwardCompounds that contain both carbonyl and alcohol functional groups are often more stable as cyclic hemiacetals or cyclic acetals than as open-chain compounds. Examples of several of these are shown. Deduce the structure of the open-chain form of each.arrow_forwardGive the organic product(s) formed in each of the following reactions. When necessary, draw a 3D representation for the product molecule showing its correct configuration.arrow_forward
- NH4Cl is sometimes preferred instead of HCl or H2SO4 for “acid” work-up after Grignard reactions, particularly when the expected and desired product is a tertiary alcohol: why?arrow_forwardMolecules with more than one alcohol group can react with thionyl chloride in a way that is different from that of single alcohols. Consider the example below. Provide a reasonable structure for product A.arrow_forwardWrite the major carbon containing product. Write optically active or racemic and if the reaction is E1, E2, SN1 or SN2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





