
(a)
Interpretation: The structure of the major product formed from acid-catalyzed hydration of
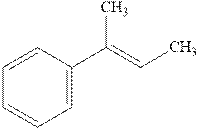
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
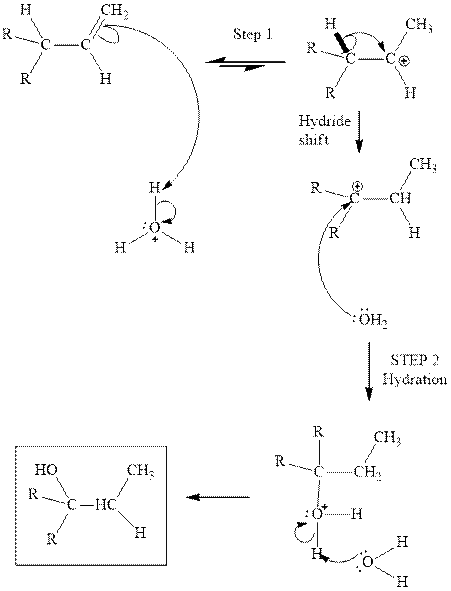
(b)
Interpretation: The structure of the major product formed fromacid-catalyzed hydration alkene indicated should be written.
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
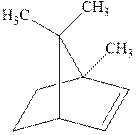
(c)
Interpretation: The structure of the major product formed from acid-catalyzed hydration alkene indicated should be written.
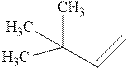
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
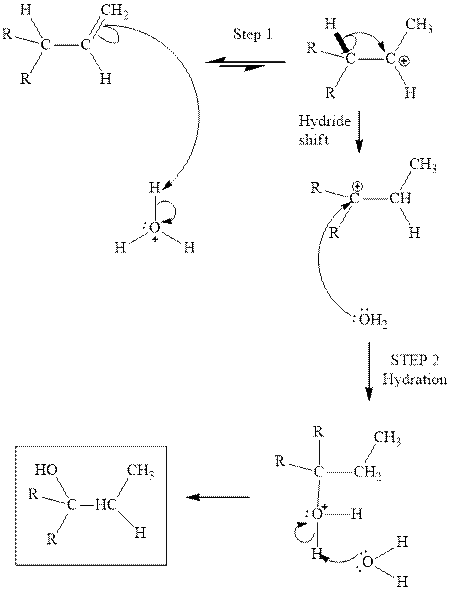
(d)
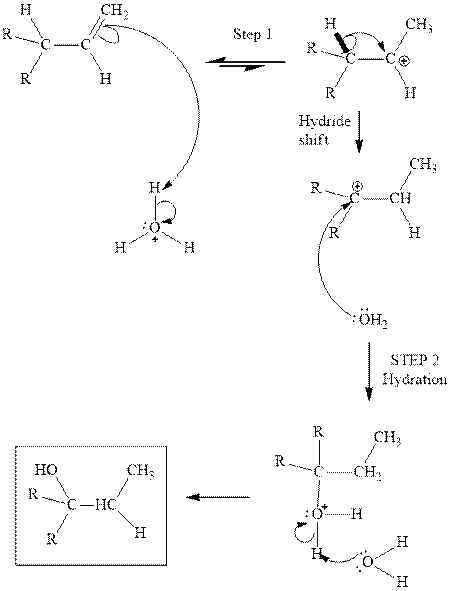
Interpretation: The structure of the major product formed from acid-catalyzed hydration of alkene indicated should be written.
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
in the first stage. In the second stage water itself acts as a nucleophile and another water abstracts a proton to give final hydration product as illustrated below.
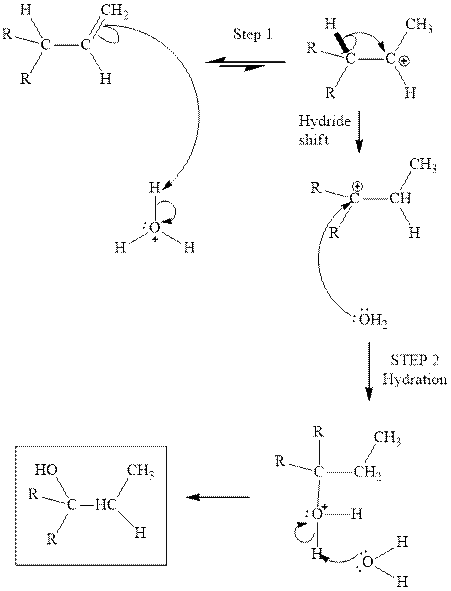
(d)
Interpretation: The structure of the major product formed from acid-catalyzed hydration alkene indicated should be written.
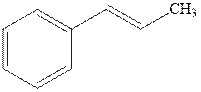
Concept introduction:Acid-catalyzed hydration is the electrophilic addition of water. The reactive species that act as a catalyst is
in the first stage. In the second stage water itself acts as a nucleophile and another water abstracts a proton to give final hydration product as illustrated below.
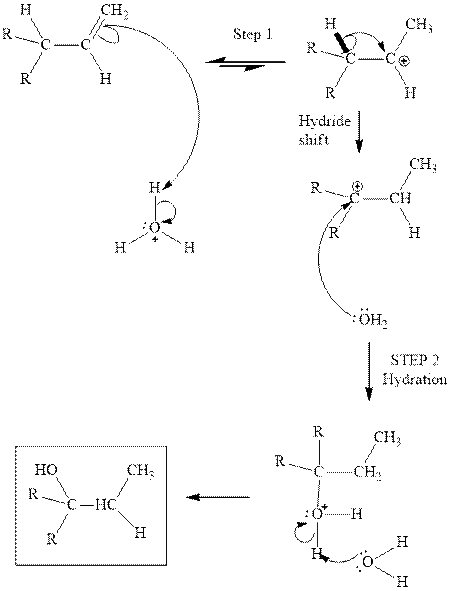
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- State and useMarkovnikov's rule and a displayed/structural formula tosuggest the dominant product when hydrogen chloride reacts with 2-methylbut-2-ene. Provide the IUPAC name of the product obtainedarrow_forward10. What is the structure of the appropriate alkene needed for the synthesis of the given alcohol by oxymercuration-demercuration?arrow_forwardWrite the products (a and c) or the necessary reagents (b, d and e) in the following reaction:arrow_forward
- 1-pentanol + NaBr/H2SO4 -> 1-bromopentane Is this substitution reaction reversible? In other words, would one expect to obtain an equilibrium mixture of the alcohol and bromoalkene at the end of the reaction of would one expect this reaction to go to completion? Justify your answer?arrow_forwardWrite equations in the synthetic direction for the preparation of 5,6-dicyanobicyclo[2.2.2]oct-2-ene from cyclohexanol and any necessary organic or inorganic reagents.arrow_forwardSeveral oxidizing reagents for alcohols were described in this chapter. Suggest one for each of the following oxidations.arrow_forward
- Write the structure of the major organic product formed in the reaction of each of the following with hydrogen bromide in the absence of peroxides and in their presence. (a) 1-Pentene (b) 2-Methyl-2-butene (c) 1-Methylcyclohexenearrow_forward1. In the reactions involving the three isomeric alcohols with the formula C4H9OH, describewhat each of the following tests showed about reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols.• the test with neutral KMnO4• the test with concentrated HCl2. Predict how the fourth alcohol with the formula C4H10O would react if tested with:• 0.01 M KMnO4• concentrated HCl at room temperatureExtend FurtherUse your observations of the solutions formed in the previous experiments and yourunderstanding of alcohols to complete a table like the one shown below. Research the meltingand boiling points to verify your answers.arrow_forwardMolecules with more than one alcohol group can react with thionyl chloride in a way that is different from that of single alcohols. Consider the example below. Provide a reasonable structure for product A.arrow_forward
- Begin by classifying the carbon atoms on either side of the oxygen atom. Propose the types of alcohol and halide which can be used for Williamson ether synthesis.arrow_forwarda) Write chemical equation including reagent(s) and condition(s) for preparation of 2-pentyne from ethyne.b) Determine the chemical equation for hydration of butyne including all reagent(s),condition(s) and intermediate(s).arrow_forwardWrite the corresponding products or reagents where necessary to obtain the products.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





