
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.15B, Problem 12.10P
Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar but distinctive mass spectra.
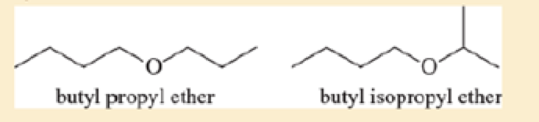
Both compounds give prominent peaks at m/z 116, 73, 57, and 43. But one compound gives a distinctive strong peak at 87, and the other compound gives a strong peak at 101. Determine which compound gives the peek at 87 and which one gives the peak at 101. Propose fragmentations to account for the ions at m/z 116, 101, 87, and 73.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar butdistinctive mass spectra.O Obutyl propyl ether butyl isopropyl etherBoth compounds give prominent peaks at m>z 116, 73, 57, and 43. But one compoundgives a distinctive strong peak at 87, and the other compound gives a strong peak at 101.Determine which compound gives the peak at 87 and which one gives the peak at 101.Propose fragmentations to account for the ions at m>z 116, 101, 87, and 73.
Identify the structure of the compound a and b having the following IR, 1H NMR spectra (integrals and splitting are shown in the boxes).
1. What is the element of unsaturation of the molecular formula? Show the math you use:
2. What are the functional groups present in this molecule? Show all of them below:
3.Draw at least two possible structures that have the required element of unsaturation as well as the observed functional groups
4.Based on the 1H NMR above, what molecular fragmentations do you see:
5.Draw your final decision of the structure below
Compounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1.
1.Based on the information provided, determine the structure of compounds B and C.
2.Assign all peaks in 1H NMR spectrum of compounds B and C.
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The ___________ functional group will have a sharp, dagger-like absorbance at 2220-2250 cm-1 on its IR spectrum (and an odd-numbered molecular mass (M+) on its mass spectrum). alkyne nitrile aldehyde alkene carbolxylic acidarrow_forwardAssume that you have a compound with the formula C4H8O. a) How many double bonds and/or rings does your compound contain? b) If your compound shows an infrared absorption peak at 1715 cm-1, what functional group does it have? c) If your compound shows a single 1H NMR absorption peak at 2.1 δ, what is its structure?arrow_forwardFor the following compound: C3H5BrO2 (Molecular Weight = 152) -Calculate the degrees of unsaturation -Use the IR and NMR to determine the structure for your compound -All 1H NMR peaks (number of signals, chemical shift, integration and splitting)-All 13C NMR peaks (number of signals and chemical shift)-All IR peaks that are important (no need to look into the fingerprint region!)-Your proposed structure of the compound (C3H5BrO2) and how it matches with the spectra (images attached)arrow_forward
- Propose a structure consistent with following set of spectral data: C3H6Br2: IR peak at 3000–2850 cm−1; NMR (ppm): 2.4 (quintet) 3.5 (triplet)arrow_forwardEXPLAIN differences in fragmentation in isomers The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively.arrow_forwardAlthough benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of substituted benzenes absorb either upfield or downfield from this value depending on the substituent. Explain the observed values for the carbon ortho to the given substituent in the monosubstituted benzene derivatives X and Y.arrow_forward
- Although benzene itself absorbs at 128 ppm in its 13C NMR spectrum, the carbons of substituted benzenes absorb either upeld or downeld from this value depending on the substituent. Explain the observed values for the carbon ortho to the given substituent in the monosubstituted benzene derivatives X and Y.arrow_forwardInfrared Interpretation – interpret all absorptions in the 4000-1400 cm-1 region of the IR spectra of 2-methyl-4-heptanol. Label the recorded IR spectra and provide an indication of the impurities present, if any.arrow_forwardcompound with the molecular formula C7H9N exhibits IR bands at 3450 cm-1 (medium, doublet), and 855 cm-1 (strong) and shows the following major mass spectral signals (m/z): 106 (base); 107 (M+, about 70% of base), 91 (40% of base), and 77 (about 20% of base). Deduce a reasonable structure from this data.arrow_forward
- Propose a structure consistent with each set of data.a. a compound that contains a benzene ring and has a molecular ion at m/z = 107b. a hydrocarbon that contains only sp3 hybridized carbons and a molecular ion at m/z = 84c. a compound that contains a carbonyl group and gives a molecular ion at m/z = 114d. a compound that contains C, H, N, and O and has an exact mass for the molecular ion at 101.0841arrow_forwardPropose a structure consistent with each set of data.a.) C9H10O2: IR absorption at 1718 cm−1 b.) C9H12: IR absorption at 2850–3150 cm−1arrow_forwardFollowing are IR and 1H-NMR spectra of compound D. The mass spectrum of compound D shows a molecular ion peak at m/z 136, a base peak at m/z 107, and other prominent peaks at m/z 118 and 59. Q.) Propose structural formulas for ions in the mass spectrum at m/z 118, 107, and 59.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY