
Concept explainers
(a)
Interpretation:
Themonosaccharides that are formed when the below disaccharide (a)is hydrolyzed should be drawn.
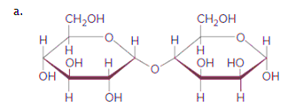
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that hold the monosaccharides together implies hydrolysis of disaccharide breaks the C-O glycosidic linkage and results in the formation of two monosaccharides.
(b)
Interpretation:
Themonosaccharides that are formed when the below disaccharide (b) is hydrolyzed should be drawn.
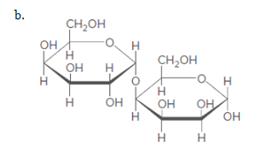
Concept introduction:
When disaccharides are hydrolyzed, a water molecule is added between the glyosidic bond that hold the monosaccharides together implies hydrolysis of disaccharide breaks the C-O glycosidic linkage or ether bonds and results in the formation of two monosaccharides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
General, Organic, and Biological Chemistry - 4th edition
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





