
Concept explainers
(a)
Interpretation:
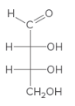
For the given monosaccharide, all the chirality centers should be labeled. The monosaccharide should be classified as D or L. The enantiomers should be drawn.
Concept Introduction:
Chirality center is an atom which has four different groups attached to it.
The D and L sugars are classified based on the position of OH group on the chirality center which is farthest from the carbonyl group. If the OH group is on the right side, it is a D sugar. If the OH group is on left side it is a L sugar.
An enantiomer is one of the two stereoisomers which are the mirror image of each other and these enantiomers are non-superimposable.
(b)
Interpretation:
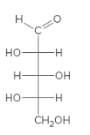
For the given monosaccharide, all the chirality centers should be labeled. The monosaccharide should be classified as D or L. The enantiomers should be drawn.
Concept Introduction:
Chirality center is an atom which has four different groups attached to it.
The D and L sugars are classified based on the position of OH group on the chirality center which is farthest from the carbonyl group. If the OH group is on the right side, it is a D sugar. If the OH group is on left side it is a L sugar.
An enantiomer is one of the two stereoisomers which are the mirror image of each other and these enantiomers are non-superimposable.
(c)
Interpretation:
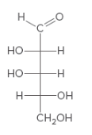
For the given monosaccharide, all the chirality centers should be labeled. The monosaccharide should be classified as D or L. The enantiomers should be drawn.
Concept Introduction:
Chirality center is an atom which has four different groups attached to it.
The D and L sugars are classified based on the position of OH group on the chirality center which is farthest from the carbonyl group. If the OH group is on the right side, it is a D sugar. If the OH group is on left side it is a L sugar.
An enantiomer is one of the two stereoisomers which are the mirror image of each other and these enantiomers are non-superimposable.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Which one is b-D glucose and which one is a-L glucose ? 2. Which one is b-fructose and which a-L fructose?arrow_forwardIn the D isomers of monosaccharides, the hydroxyl group on the stereogenic carbon Select one: a. angles upwards at 90 degrees b. does not exist c. points to the left d. points to the rightarrow_forwardThe following trisaccharide derivative is important to human health.What ever the A ring is called, it is linked to the B ring as ?-O-(A Ringyl)-B ringly-C ring?arrow_forward
- a) Draw Haworth projections of both - and -anomers of D-fructose. Indicate which carbon is the anomeric carbon.b) Sucrose is a disaccharide made up of a molecule of D-fructose and D-glucose. Draw the structure of sucrose clearly indicating the linkage between the two monosaccharides and its biological significance.c) Tollen’s reagent is a very mild oxidizing agent which normally oxidize aldehydes but not ketones. However, both glucose and fructose give positive results with Tollen’s reagent and are classified as reducing sugars. Explain how fructose can also give positive results with Tollen’s reagent (illustrate using structures).arrow_forwardThe following trisaccharide derivative is important to human health.The A ring is?arrow_forwardDraw a chair conformation for the a form of a disaccharide in which two units of d-glucopyranose are joined by a b-1,3-glycosidic bond.arrow_forward
- A) Define "reducing sugar." (b) Sucrose is a disaccharide composed of glucose and fructose (Glc(1 → 2)Fru). Explain why sucrose is not a reducing sugar, even though both glucose and fructose are.arrow_forwardAnswer the following questions about monosaccharide B.a. Draw the β anomer of B in a Haworth projection.b. Draw the α anomer of B in a three-dimensional representation using a chair conformation.c. What products are formed when B undergoes the Kiliani–Fischer synthesis?d. What product is formed when B is treated with NaBH4 in CH3OH?e. Draw the disaccharide formed when two molecules of B are joined by a 1→4-β-glycosidic linkage.arrow_forward1. Construct the two enantiomeric forms/structure of the following monosaccharides and designate the handedness of each using D, L system: a. Xylose b. Psicose c. Mannose d. Ribulosearrow_forward
- Draw the chair conformation of a monosaccharide that is anomeric with α-D-glucopyranosearrow_forward(A) Is the trisaccharide a reducing sugar? (B) Identify the type of glycosidic linkage of the colored bond.arrow_forwardConsider the tetrasaccharide stachyose drawn below. Stachyose is found in white jasmine, soybeans, and lentils. Because humans cannot digest it, its consumption causes flatulence.a. Label all glycoside bonds.b. Classify each glycosidic linkage as αα or ββ and use numbers to designate its location between two rings (e.g., 1→→4-ββ)-c. What products are formed when stachyose is hydrolyzed with H33O++?d. Is stachyose a reducing sugar?e. What product is formed when stachyose is treated with excess CH33I, Ag22O?f. What products are formed when the product in (e) is treated with H33O++?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

