
Concept explainers
Interpretation:
The curved arrow notation is to be drawn for the proton transfer between ammonia
Concept introduction:
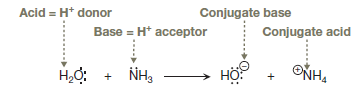
In a proton transfer reaction, a proton is transferred from a Bronsted–Lowry acid (proton donor) to a Bronsted–Lowry base (proton acceptor) in a single elementary step in which one bond is broken and another is formed simultaneously. The conjugate acid is the species that the base becomes after gaining a proton, and the conjugate base is the species that the acid becomes after losing a proton. The curved arrow notation shows the movement of valence electrons, not atoms. Each double-barbed curved arrow shows the movement of two valence electrons. To represent bond breaking, the tail of the arrow originates from the center of a bond whereas to represent bond formation, the head of the arrow points to an atom that forms the new bond, that is,
Answer to Problem 6.1P
The curved arrow notation for the proton transfer between ammonia
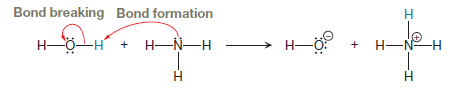
Explanation of Solution
The given proton transfer reaction is between ammonia
The bond breaking and bond formation involves only valence electrons, so first, we need to draw all valence electrons in the given two reactants. From this, it is clearly seen which electrons are involved in the reaction, both from the reactants and from the products as shown below:
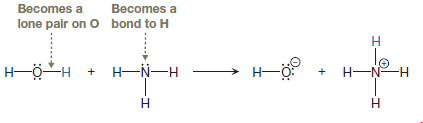
The appropriate movement of these valence electrons is shown by using curved arrow notations. One curved arrow is to be drawn from the lone pair on N to the H on water to illustrate the formation of

The curved arrow notation for the proton transfer of the given reaction is drawn on the basis of movement of valence electrons involved in bond breaking and bond formation.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- The following are equivalent ways of asking about the acidity of an H atom: • What is the most acidic H on the molecule? • Which H is associated with the published pKa value? • Which H on the molecule is easiest to remove? • Which H on the molecule takes the least energy to remove? • Which bond to an H is most polarized? • For which H atom is removal least uphill in energy? • Which bond to an H atom, when broken, results in the lowest PE conjugate base? We will often find the last of these questions is easiest to answer. To do this, find all the different Hatoms on the molecule, and draw all possible conjugate bases.Only the lowest-energy one is the “real” conjugate base. Identify this structure, and you have found the most acidic H. Use this strategy to find the most acidic H on each of the following molecules. Note: Each structure hasat least three different kinds of H’s, so draw at least three unique conjugate bases for each.arrow_forwardAnswer true or false to the following statements about the mechanism of acid-base reactions. (a) The acid and base must encounter each other by a collision in order for the proton to transfer. (b) All collisions between acids and bases result in proton transfer. (c) During an acid-base reaction the lone pair on the base fills the A-H antibonding sigma orbital.arrow_forwardComplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forward
- Draw the structure of the conjugate base of water. (Note that it does not appear in Figure 4.11).arrow_forwardSummarize the relationship between pKa and base strength by completing the followingsentences: a. For a given base, the higher the pKa of its conjugate acid, the stronger or weaker the base. b. For a given base, the lower the pKa of its conjugate acid, the stronger or weaker the base.arrow_forwardList the following bases in order of their decreasing strength strongest base first: CN,H2O,HSO3,ClO,Cl.arrow_forward
- Identify the conjugate acid/conjugate base pairs for the structure below. See picture attached.arrow_forwardDecide which compound is the acid and which is the base, and draw the products of each proton transfer reaction.arrow_forwardHow do you know which is the acid and which is the base in a proton transfer reaction? Give generalizations ?arrow_forward
- Using the data in the table, which of the conjugate bases below is the weakest base?arrow_forwardDetermining the Acid, Base, Conjugate Acid, and Conjugate Base in a Reaction Label the acid and base, and the conjugate acid and base, in the following reaction. Use curved arrow notation to show the movement of electron pairs.arrow_forwardHi , can you help me to answer this question .. can you draw curved arrows to move a proton from acid to the base .. Identify acid , base , conjugate acid and conjugate base also draw the product of proton transfer.. I need the full scheme answe so that i can understand this topic better.. Thank youuarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



