
(a) Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
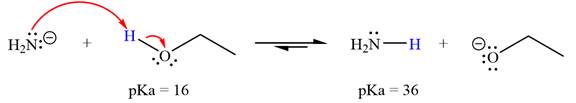
Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving
Explanation of Solution
The reaction of the acetate ion
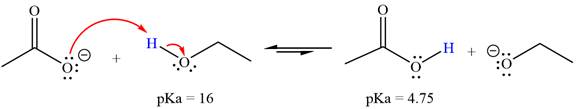
Acetic acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving chloride ion
Explanation of Solution
The reaction of chloride ion
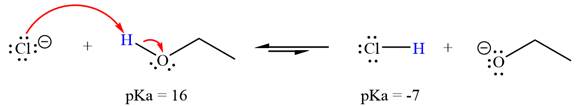
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving phenoxide ion (
Explanation of Solution
The reaction of phenoxide ion (
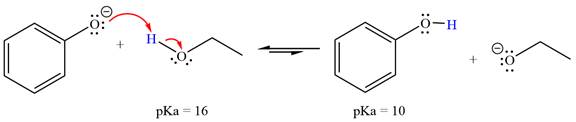
Phenol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is a suitable solvent for a reaction involving
Explanation of Solution
The reaction of cyanide ion
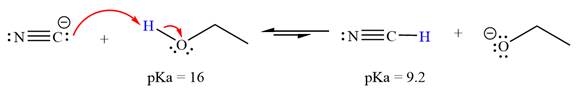
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
(f)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanol as a solvent with respect to leveling effect.
Concept introduction:
The solvent affects the properties of bases and acids. This effect is referred as leveling effect. For an acid-base reaction, the basicity of the solvent levels or limits the strength of the strong acid. Similarly, the acidity of the solvent levels the strength of the strong base. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.45P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of propyl group
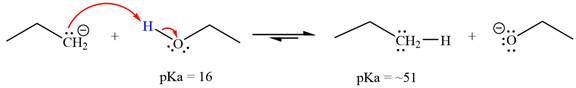
Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- With reference to its molecular orbital diagram, state –with justification – how CO can act as a nucleophile, and whether it will attack its reacting partner through the carbon or oxygen atomarrow_forwardWhy is this Beckmann rearrangemnt useful for the synthesis of acetaminophen?arrow_forwardA fifth compound was studied but not included in the table (see paragraph II) 1,1-dibromoethylbenzene. This compound gave only substitution products following an mechanism. Suggest why this compound would react preferably via SN1 mechanism and not SN2? Give structures to support your conclusion.arrow_forward
- Give only typing answer with explanation and conclusion What is the main product formed in the Friedel-Crafts alkylation reaction of benzene with 1-butene and AlCl3?arrow_forwardProvide the amine and coupling component that can be used as starting materials to synthesize the following dyesarrow_forwardDiscuss the regioselectivity of the compound equation below, including in your answer the terms: radical plusstable, homolytic breakage, main product.arrow_forward
- Give the major organic product of the reaction.arrow_forwardAssuming you are a chemist and you need to extract ethanolic acid in a benzene solution. Propose and briefly discuss how to extract the ethanolic acid in the benzene solution. Include the reactions involved in each step, if any.arrow_forwardProvide the curved-arrow mechanism to account for the following nucleophilic addition- elimination reaction.arrow_forward
- Please provide bond energy dissociation accurately for these 3 bonds for the below structure (with reference must be from a book or an article which I can find please): between carbon - carbon within the aromatic ring; between carbon - carbon between two aromatic rings; and between carbon - hydrogen within the aromatic ring. Thanks so much for your help!arrow_forwardWill adding carboxylic acid added in excess and the alcohol as the limiting reagent maximize the yield in synthesizing esther?arrow_forwardPlease Don't provide handwritten solution ..... Predict the major products of this organic reaction.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
