
Concept explainers
(a)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
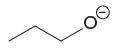
The stronger base in the given pair of species is
Explanation of Solution
The given pair of species is

The second species has negative charge whereas the first species is uncharged. A negatively charged species is less stable than the uncharged species; hence the negatively charged species is more basic than the uncharged species. Therefore, the stronger base in given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(b)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
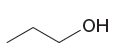
The stronger base in the given pair of species is
Explanation of Solution
The given pair of species is

The first species is neutral, and the second is positively charged. The neutral species can easily abstract protons as it has two lone pairs whereas the second species has no lone pair to abstract a proton. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(c)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
![]()
Explanation of Solution
The given pair of species is

In the first species, the negative charge is on the
![]()
The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(d)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
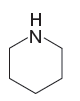
The stronger base in the given pair of species is
Explanation of Solution
The given pair of species is
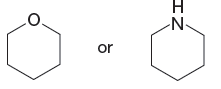
The first species has an oxygen atom and second species has an N atom. The nitrogen atom is less electronegative than oxygen and can easily donate a lone pair. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(e)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
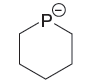
Explanation of Solution
The given pair of species is
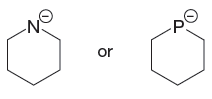
The first species has a negatively charged nitrogen atom whereas the second species has a negatively charged P atom. The phosphorus atom is less electronegative than nitrogen. The species having a negative charge on less electronegative atom is more basic. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(f)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
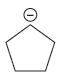
Explanation of Solution
The given pair of species is
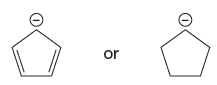
The first species is more stable due to resonance; it shows the delocalization of the negative charge and makes the species more acidic, whereas, the second species does not show the resonance effect, making it more basic than the first species. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(g)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
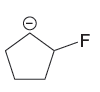
Explanation of Solution
The given pair of species is
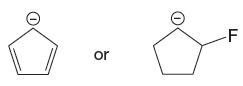
The first species is more stable due to resonance; it shows the delocalization of the negative charge and makes the species more acidic, whereas the second species does not show resonance effect, making it more basic than the first species. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(h)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
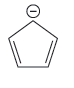
Explanation of Solution
The given pair of species is
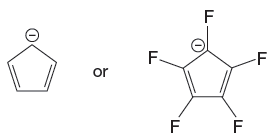
Both the species have the resonance effect but presence of five electronegative F atoms in the second species makes it more stable and therefore more acidic than the first species. Therefore, the stronger base in the given pair of species is

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
(i)
Interpretation:
The stronger base from the given pair of species is to be predicted.
Concept introduction:
According to the Bronsted Lowry theory, a base is the species that accepts a proton by donating its lone pair of electrons. A negatively charged species is less stable than uncharged species; hence the negatively charged species is more basic. The negative charge on the less electronegative atom makes it a stronger base. Resonance can stabilize a negatively charged species and make it a weaker base. Electron donating group, which is less electronegative than hydrogen, stabilizes the positive charge but destabilizes a nearby negative charge and makes the species more basic. The neutral species may easily abstract protons due to the lone pair and may become more basic than the positively charged species. A positive charge is energetically favored on the atom with the lower effective electronegativity, i.e.,
Answer to Problem 6.64P
The stronger base in the given pair of species is
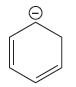
Explanation of Solution
The given pair of species is

The negative charge on the first species is in complete conjugation, making it more stable and increasing the acidity. The negative charge on the second species shows conjugation, but there is no

The stronger base from the given pair of species is predicted on the basis of factors affecting charge stability.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- For each molecule below, draw the conjugate acid or conjugate base or both if the molecule hasboth a conjugate acid and a conjugate base (e.g., water).arrow_forwardThe following are equivalent ways of asking about the acidity of an H atom: • What is the most acidic H on the molecule? • Which H is associated with the published pKa value? • Which H on the molecule is easiest to remove? • Which H on the molecule takes the least energy to remove? • Which bond to an H is most polarized? • For which H atom is removal least uphill in energy? • Which bond to an H atom, when broken, results in the lowest PE conjugate base? We will often find the last of these questions is easiest to answer. To do this, find all the different Hatoms on the molecule, and draw all possible conjugate bases.Only the lowest-energy one is the “real” conjugate base. Identify this structure, and you have found the most acidic H. Use this strategy to find the most acidic H on each of the following molecules. Note: Each structure hasat least three different kinds of H’s, so draw at least three unique conjugate bases for each.arrow_forwardConsider the following bases: a. For each base above, circle the atom/atoms with the highest PE (will release the most P.E.when a lone pair on this atom combines with an H+ ) b. Rank the bases 1 (highest P.E./strongest base) to 7 (lowest PE/weakest base), and explainyour reasoning.arrow_forward
- Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reaction. (a) (b) (c) (d)arrow_forwardFor the previous four questions, label each molecule that appears in the question or your answer asstrong acid, strong base, weak acid, or weak base.arrow_forwardSummarize the relationship between pKa and acid strength by completing the following sentences: a. The higher the pKa of an acid, the stronger or weaker the acid. b. The lower the pKa of an acid, the stronger or weaker the acid.arrow_forward
- Construct an explanation for why sulfuric acid is such a strong acid. (Note that sulfur is in thethird row of the periodic table and can have more than eight electrons.)arrow_forwardComplete each Lewis structure, draw all important resonance structures, predict a value for thebond angles requested, and explain your reasoning. a. Nitrous acid (HNO2)HONOHON=ONO= b. Enolate ion (C2H3O) HC1C2=HC2C1=arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


