
a)
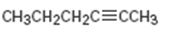
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
b)
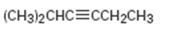
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of alkynes is an efficient method for preparing higher alkanes. Acetylene upon alkylation gives a terminal alkyne while further alkylation of a terminal alkyne leads to the formation of an internal alkyne. The actylide anion, being nucleophilic in nature, displaces the halide ion when treated with alkyl halide and gets itself attached to the alkyl group to yield a terminal alkyne. Only primary alkyl halides can be used in the reaction because when secondary and tertiary alkyl halides are used elimination of a hydrogen halide occurs instead of substitution.
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
c)
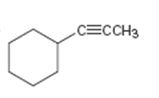
Interpretation:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained are to be shown. If two routes are possible both has to be listed.
Concept introduction:
The alkylation of alkynes is an efficient method for preparing higher alkanes. Acetylene upon alkylation gives a terminal alkyne while further alkylation of a terminal alkyne leads to the formation of an internal alkyne. The actylide anion, being nucleophilic in nature, displaces the halide ion when treated with alkyl halide and gets itself attached to the alkyl group to yield a terminal alkyne. Only primary alkyl halides can be used in the reaction because when secondary and tertiary alkyl halides are used elimination of hydrogen halide occurs instead of substitution.
To show:
The terminal alkyne and the alkyl halide from which the compound shown can be obtained. Further to list both routes if two routes are possible.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Organic Chemistry

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

