
(a)
Interpretation: The freezing point of the liquid has to be given.
Concept Introduction: Heating curves represents the change in temperature as a substance is heated. Cooling curves represents the change in temperature as a substance is cooled. Heating and cooling curves have horizontal flat parts that show the phase changes.
(a)
Answer to Problem 12STP
The freezing point of the liquid is
Explanation of Solution
The given heating-cooling curve is,
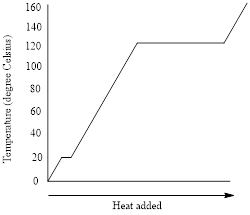
Figure 1: Heating-cooling curve of the liquid
The heating curve (addition of energy) goes from left to right in the above graph and the cooling curve (removal of energy) goes from right to left.
The flat portions of the curve represent the changes in phases and the slope of the curve represents the changes in temperature.
For the freezing point of a liquid, the cooling curve is considered. The freezing point is the temperature at which liquid turns into solid. The freezing point of the liquid in the given heating-cooling curve is
(b)
Interpretation: The boiling point of the liquid has to be given.
Concept Introduction: Heating curves represents the change in temperature as a substance is heated. Cooling curves represents the change in temperature as a substance is cooled. Heating and cooling curves have horizontal flat parts that show the phase changes.
(b)
Answer to Problem 12STP
Explanation of Solution
The given heating-cooling curve is,
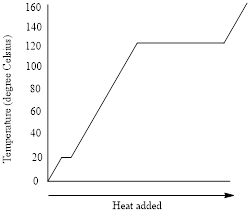
Figure 1: Heating-cooling curve of the liquid
The heating curve (addition of energy) goes from left to right in the above graph and the cooling curve (removal of energy) goes from right to left.
The flat portions of the curve represent the changes in phases and the slope of the curve represents the changes in temperature.
For the boiling point of the liquid, the heating curve is considered. The boiling point is the temperature at which liquid changes to vapor phase by the addition of heat. The boiling point of the liquid in the given heating-cooling curve is
(c)
Interpretation: The property (heat of fusion or heat vaporization) that is greater has to be given and the reason has to be explained.
Concept Introduction:
The heat of fusion is the energy that is needed to melt one mole of solid to liquid at a constant temperature.
The heat of vaporization is the energy that is needed to convert one mole of liquid to a gas at a constant temperature.
(c)
Answer to Problem 12STP
The heat of vaporization is greater because the energy needed to convert the molecules from liquid to gas is much greater than that of the energy needed to convert the molecules from solid to liquid.
Explanation of Solution
The heat of fusion is the energy that is needed to melt one mole of solid to liquid at a constant temperature.
The heat of vaporization is the energy that is needed to convert one mole of liquid to a gas at a constant temperature.
In solids, the molecules are closely packed and they have a strong intermolecular force of attraction. The strong intermolecular forces cause the substance to have a structure in which the molecule has little freedom to move.
In liquids, the molecules are closely packed but less than that of solid. They have more freedom and the intermolecular forces are weaker than that of solid.
In gases, the molecules are far apart from each other and they have the weakest intermolecular forces.
When a solid is heated into a liquid, the kinetic energy of its molecules increases, move them further apart until the intermolecular forces are decreased to allow it to flow freely.
When a liquid is heated into vapor, the kinetic energy of the molecules is increased to a point where there is no intermolecular force of attraction between the molecules.
The heat of vaporization is greater because the energy needed to convert the molecules from liquid to gas is much greater than that of the energy needed to convert the molecules from solid to liquid.
Chapter 14 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





