
Concept explainers
Interpretation: The difference between intramolecular and intermolecular forces has to be explained.
Concept introduction: On the molecular level, atoms and molecules interact with each other through
- Intermolecular bonds
- Intramolecular bonds
Intramolecular bonds are those bonds that strongly hold the atoms in a molecule together.
Intermolecular bonds are those bonds that strong hold the atoms on different molecules together.
Answer to Problem 18A
When the forces occur between different molecules, they are called as intermolecular forces.
When the force acts inside the same molecules, they are called as intramolecular forces.
Explanation of Solution
On the molecular level, atoms and molecules interact with each other through chemical bonds. Bonds can be classified into two types called as,
- Intermolecular bonds
- Intramolecular bonds
Intramolecular bonds are those bonds that strongly hold the atoms within a molecule together, and the forces acting are called as intramolecular forces.
Intramolecular forces are of three types,
- Ionic bonding
- Covalent bonding
- Metallic bonding
Intermolecular bonds are those bonds that strong hold the atoms on different molecules together and the forces acting are called as intermolecular forces.
Intermolecular forces of three types,
- Dipole-dipole attraction
- Hydrogen bonding
- London dispersion forces
The below diagram is an example of intermolecular and intramolecular forces.
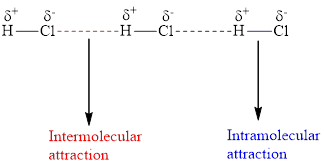
Intermolecular forces occur between different molecules, whereas intramolecular forces occur inside the same molecule.
Chapter 14 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





