
Interpretation: The meaning of different portion in the cooling curve of water has to be discussed.
Concept introduction: Heating curves represents the change in temperature as a substance is heated. Cooling curves represents the change in temperature as a substance is cooled. Heating and cooling curves have horizontal flat parts that shows the phases changes.
Answer to Problem 15A
The phase changes are given by the flat portions of the curve whereas, the slope represents the changes in temperature.
Explanation of Solution
The heating-cooling curve of water is,
The x-axis represents the amount of heat added at constant rate and the temperature in
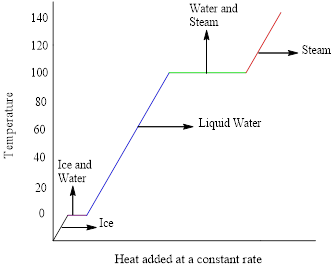
Figure 1: Heating-cooling curve of water
The heating curve (addition of energy) goes from left to right in the above graph and the cooling curve (removal of energy) goes from right to left.
The flat portions of the curve represent the changes in phases and the slope of the curve represents the changes in temperature.
The changes in phase are given by the flat portions of the curve whereas, the slope represents the changes in temperature.
Chapter 14 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





