
Interpretation: The explanation for the difference between the boiling point of methane and ammonia needs to be provided.
Concept Introduction: The strength of intermolecular forces present between the molecules influences the properties of the substance like boiling point, freezing point, etc. If the strength of the intermolecular forces is strong, then it results in a higher boiling point and low vapor pressure of the liquid.
Explanation of Solution
The structures of ammonia,
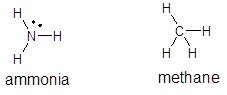
Methane is a non-polar molecule, whereas ammonia is a polar molecule. The force of attraction between polar molecules are greater than those of non-polar molecule, so methane being non-polar will possess less attraction between methane molecules, but ammonia molecules possess polar
Due to the presence of a nitrogen atom in ammonia, the formation of intermolecular hydrogen bonding takes place in which the N atom of one molecule of ammonia is bonded to the H atom of another molecule of ammonia. The hydrogen bonding in
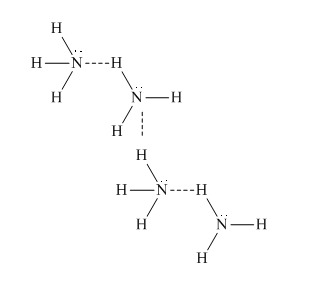
Hence, due to hydrogen bonding in ammonia, the ammonia molecule has a higher boiling point than methane.
Chapter 14 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





