
Interpretation:
The heating curve is to be constructed from the given data.
Concept introduction:
The temperature at which the solid and liquid states of pure substance exits as equilibrium are called melting point of substance. As heat provide to as solid substance, temperature increases until melt itself. More heat will convert solid to liquid without temperature change. And the heat is to be required to convert unit mass of solid to liquid are called heat of fusion.
The temperature at which vapor pressure of liquid is equal to the atmospheric pressure (surrounding pressure) called boiling point of substance. At this condition, addition of heat results to transform the liquid to vapor without raising temperature. The heat absorbed by the one mole of substance to convert from a liquid to gas physical state is called molar heat of vaporization.
Answer to Problem 21A
The heating curve of substance X is,
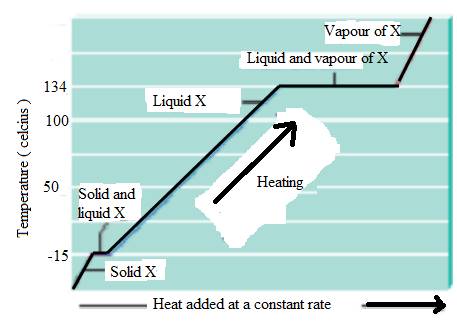
Explanation of Solution
The state of substance change is physical change. Heat energy provides which increases the vibration motion of the molecule, and increases the kinetic energy of substance. The substance X with melting at
The substance X is reached at
Boiling process required more heat than melting.
Chapter 14 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





