
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 23.100CHP
Interpretation Introduction
Interpretation:
The structure of the ester given in the problems statement should be drawn.
Concept Introduction:
Fischer esterification: Ester can be synthesised from the reaction of Alcohols and carboxylic acid in the presence of acid catalyst and it is called Fischer esterification.
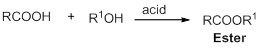
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An isomer of C6H12O could contain a carboxylic acid. True or false?
In an esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic conditions to form an ester. Draw the structure of the ester product in the reaction between pentanoic acid and 1‑propanol.
What happens when the following substance reacts with water in the presence of concentrated sulfuric acid?
a)
An alkyne is produced.
b)
An alcohol is produced.
c)
The substance is converted to its cis isomer.
d)
The substance is converted to its trans isomer.
Chapter 23 Solutions
General Chemistry: Atoms First
Ch. 23.1 - Prob. 23.1PCh. 23.1 - Prob. 23.2PCh. 23.1 - Prob. 23.3PCh. 23.1 - Prob. 23.4CPCh. 23.2 - Prob. 23.5PCh. 23.2 - Prob. 23.6PCh. 23.3 - Prob. 23.7PCh. 23.3 - Prob. 23.8PCh. 23.3 - Prob. 23.9CPCh. 23.4 - Prob. 23.10P
Ch. 23.4 - Prob. 23.11PCh. 23.4 - Prob. 23.12PCh. 23.4 - Prob. 23.13PCh. 23.5 - Prob. 23.14PCh. 23.5 - Draw structures corresponding to the following...Ch. 23.6 - Draw structures corresponding to the following...Ch. 23.6 - Write the products from reaction of the following...Ch. 23.6 - Reaction of Br2/FeBr3 with toluene (methylbenzene)...Ch. 23.7 - Prob. 23.19PCh. 23.8 - Draw structures corresponding to the following...Ch. 23.8 - Prob. 23.21PCh. 23.8 - Prob. 23.22CPCh. 23.10 - Prob. 23.23PCh. 23.10 - Prob. 23.24PCh. 23.10 - Prob. 23.25PCh. 23.11 - Prob. 23.26PCh. 23.12 - Show the structure of glyceryl trioleate, a fat...Ch. 23.13 - Prob. 23.28PCh. 23.13 - Prob. 23.29PCh. 23.13 - Prob. 23.30PCh. 23.13 - Prob. 23.31CPCh. 23.13 - Prob. 23.32PCh. 23 - Prob. 23.33CPCh. 23 - Prob. 23.34CPCh. 23 - Prob. 23.35CPCh. 23 - Prob. 23.36CPCh. 23 - Prob. 23.37CPCh. 23 - Prob. 23.38CPCh. 23 - Prob. 23.39CPCh. 23 - Prob. 23.40SPCh. 23 - Prob. 23.41SPCh. 23 - Prob. 23.42SPCh. 23 - Prob. 23.43SPCh. 23 - Prob. 23.44SPCh. 23 - Prob. 23.45SPCh. 23 - Prob. 23.46SPCh. 23 - Prob. 23.47SPCh. 23 - Prob. 23.48SPCh. 23 - What is wrong with each of the following...Ch. 23 - What are the IUPAC names of the following alkanes?Ch. 23 - Prob. 23.51SPCh. 23 - Prob. 23.52SPCh. 23 - Prob. 23.53SPCh. 23 - Prob. 23.54SPCh. 23 - Prob. 23.55SPCh. 23 - Prob. 23.56SPCh. 23 - Prob. 23.57SPCh. 23 - Prob. 23.58SPCh. 23 - Prob. 23.59SPCh. 23 - Prob. 23.60SPCh. 23 - Prob. 23.61SPCh. 23 - Prob. 23.62SPCh. 23 - Prob. 23.63SPCh. 23 - Draw structures corresponding to the following (a)...Ch. 23 - Prob. 23.65SPCh. 23 - Prob. 23.66SPCh. 23 - Prob. 23.67SPCh. 23 - Prob. 23.68SPCh. 23 - Draw and name compounds that meet the following...Ch. 23 - Prob. 23.70SPCh. 23 - Prob. 23.71SPCh. 23 - Prob. 23.72SPCh. 23 - Prob. 23.73SPCh. 23 - Prob. 23.74SPCh. 23 - Prob. 23.75SPCh. 23 - Prob. 23.76SPCh. 23 - Prob. 23.77SPCh. 23 - Prob. 23.78SPCh. 23 - Prob. 23.79SPCh. 23 - Prob. 23.80SPCh. 23 - Prob. 23.81SPCh. 23 - Prob. 23.82SPCh. 23 - Prob. 23.83SPCh. 23 - Prob. 23.84SPCh. 23 - Prob. 23.85SPCh. 23 - Prob. 23.86SPCh. 23 - Prob. 23.87SPCh. 23 - There are two isomeric fat molecules whose...Ch. 23 - Prob. 23.89SPCh. 23 - What is a nucleotide, and what three kinds of...Ch. 23 - What are the names of the sugars in DNA and RNA,...Ch. 23 - Prob. 23.92SPCh. 23 - Prob. 23.93SPCh. 23 - Prob. 23.94SPCh. 23 - Prob. 23.95SPCh. 23 - Prob. 23.96SPCh. 23 - Prob. 23.97SPCh. 23 - Prob. 23.98CHPCh. 23 - Prob. 23.99CHPCh. 23 - Prob. 23.100CHPCh. 23 - Prob. 23.101CHPCh. 23 - Write full structures for the following peptides,...Ch. 23 - Prob. 23.103CHPCh. 23 - Prob. 23.104CHPCh. 23 - Prob. 23.105CHPCh. 23 - Prob. 23.106CHPCh. 23 - Elaidic acid, a component of so-called trans fats,...Ch. 23 - Fumaric acid is an organic substance widely used...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Maleic acid and fumaric acid are the cis- and trans- isomers, respectively, of C2H2(COOH)2, a dicarboxylic acid. Draw and label their structures.arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardThe foul odor of rancid butter is caused by butyric acid, CH3CH2CH2CO2H. (a) Draw the Lewis structure and determine the oxidation number and hybridization for each carbon atom in the molecule. (b) The esters formed from butyric acid are pleasant-smelling compounds found in fruits and used in perfumes. Draw the Lewis structure for the ester formed from the reaction of butyric acid with 2-propanol.arrow_forward
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
