1 Chemistry: An Introduction 2 Measurements And Calculations 3 Matter 4 Chemical Foundations: Elements, Atoms, And Ions 5 Nomenclature 6 Chemical Reactions: An Introduction 7 Reactions In Aqueous Solutions 8 Chemical Composition 9 Chemical Quantities 10 Energy 11 Modern Atomic Theory 12 Chemical Bonding 13 Gases 14 Liquids And Solids 15 Solutions 16 Acids And Bases 17 Equilibrium 18 Oxidation–reduction Reactions And Electrochemistry 19 Radioactivity And Nuclear Energy 20 Organic Chemistry 21 Biochemistry expand_more
15.1 Solubility 15.2 Solution Composition: An Introduction 15.3 Solution Composition: Mass Percent 15.4 Solution Composition: Molarity 15.5 Dilution 15.6 Stoichiometry Of Solution Reactions 15.7 Neutralization Reactions 15.8 Solution Composition: Normality Chapter Questions expand_more
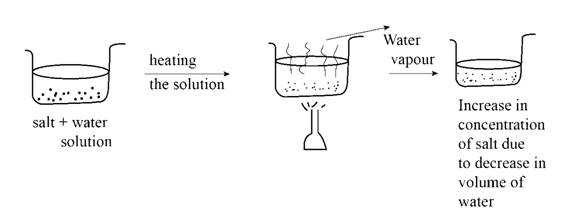
Problem 1ALQ: ou have a solution of table sail in water. What happens to the salt concentration (increases,... Problem 2ALQ: onsider a sugar solution (solution A) with concentration x. You pour one-third of this solution into... Problem 3ALQ: You need to make 150.0 mL of a 0.10 M NaCI solution. You have solid NaCl, and your lab partner has a... Problem 4ALQ: ou have two solutions containing solute A. To determine which solution has the highest concentration... Problem 5ALQ: m>5. Which of the following do you need to know to calculate the molarity of a salt solution? (There... Problem 6ALQ: onsider separate aqueous solutions of HCI and H2S04 with the same concentrations in terms of... Problem 7ALQ Problem 8ALQ: an one solution have a greater concentration than another in terms of weight percent, but a lower... Problem 9ALQ Problem 10ALQ: You have equal masses of different solutes dissolved in equal volumes of solution. Which of the... Problem 11ALQ: Which of the following solutions contains the greatest number of particles? Support your answer. .0... Problem 12ALQ: As with all quantitative problems in chemistry, make sure not to get “lost in the math.” In... Problem 13ALQ Problem 14ALQ Problem 1QAP: solution is a homogeneous mixture. Can you give an example of a gaseous homogeneous mixture? A... Problem 2QAP: ow do the properties of a nonhomogeneous (heterogeneous) mixture differ from those of a solution?... Problem 3QAP Problem 4QAP Problem 5QAP: n Chapter 14. you learned that the bonding forces in ionic solids such as NaCl are very strong, yet... Problem 6QAP: n oil spill spreads out on the surface of water, rather than dissolving in the water. Explain why. Problem 7QAP: . The “Chemistry in Focus” segment Water, Water Everywhere, Bu . . . discusses the desalinization of... Problem 8QAP Problem 9QAP Problem 10QAP Problem 11QAP: A solution is a homogeneous mixture and, unlike a compound, has composition. Problem 12QAP Problem 13QAP: How do we define the mass percent composition of a solution? Give an example of a solution, and... Problem 14QAP Problem 15QAP: Calculate the percent by mass of solute in each of the following solutions. 14 g of potassium... Problem 16QAP: Calculate the percent by mass of solute in each of the following solutions. 11 mg of calcium... Problem 17QAP Problem 18QAP Problem 19QAP: A sample of an iron alloy contains 92.1 g Fe. 2.59 g C. and Cr. Calculate the percent by mass of... Problem 20QAP: Consider the iron alloy described in Question 19. Suppose it is desired to prepare 1.00 kg of this... Problem 21QAP: An aqueous solution is to be prepared that will be 7.51% by mass ammonium nitrate. What mass of... Problem 22QAP Problem 23QAP: A solution is to be prepared that will be 4.50% by mass calcium chloride. To prepare 175 g of the... Problem 24QAP Problem 25QAP Problem 26QAP: Hydrogen peroxide solutions sold in drugstores as an antiseptic typically contain 3.0% of the active... Problem 27QAP Problem 28QAP: A solvent sold for use in the laboratory contains 0.95% of a stabilizing agent that prevents the... Problem 29QAP Problem 30QAP: A solution labeled “0.25 M AICl3” would contain _________ le(s) of Al3+ and mole(s) of Cl- in each... Problem 31QAP: What is a standard solution? Describe the steps involved in preparing a standard solution. Problem 32QAP Problem 33QAP: 33. For each of the following solutions, the number of moles of solute is given, followed by the... Problem 34QAP: 34. For each of the following solutions, the number of moles of solute is given, followed by the... Problem 35QAP: 35. For each of the following solutions, the mass of solute is given, followed by the total volume... Problem 36QAP Problem 37QAP: 37. A laboratory assistant needs to prepare 225 mL of 0.150 M CaCl2 solution. How many grams of... Problem 38QAP Problem 39QAP: 39. Standard solutions of calcium ion used to test for water hardness are prepared by dissolving... Problem 40QAP Problem 41QAP: 41. If 42.5 g of NaOH is dissolved in water and diluted to a final volume of 225 mL, calculate the... Problem 42QAP: 42. Standard silver nitrate solutions are used in the analysis of samples containing chloride ion.... Problem 43QAP Problem 44QAP Problem 45QAP Problem 46QAP Problem 47QAP Problem 48QAP: 48. What mass of solute is present in 225 mL of 0.355 M KBr solution? Problem 49QAP Problem 50QAP Problem 51QAP Problem 52QAP: 52. What volume of a 0.300 M CaCl2 solution is needed to prepare 240. mL of a 0.100 M CI- solution? Problem 53QAP Problem 54QAP Problem 55QAP Problem 56QAP Problem 57QAP Problem 58QAP Problem 59QAP Problem 60QAP: 60. Suppose 325 in L of 0.150 M NaOH is needed for your experiment. How would you prepare this if... Problem 61QAP: 61. How much water must be added w 500. mL of 0.200 M HCI to produce a 0.150 M solution? (Assume... Problem 62QAP Problem 63QAP Problem 64QAP: 64. Generally only the carbonates of the Group I elements and the ammonium ion are soluble in water:... Problem 65QAP: 65. Many metal ions are precipitated from solution by the sulfide ion. As an example, consider... Problem 66QAP: 66. Calcium oxalate, CaCO4, is very insoluble in water. What mass of sodium oxalate, Na2C2O4, is... Problem 67QAP: 67. When aqueous solutions of lead(II) ion are treated with potassium chromate solution, a bright... Problem 68QAP: 68. Aluminum ion may be precipitated from aqueous solution by addition of hydroxide ion, forming... Problem 69QAP: 69. What volume of 0.502 M NaOH solution would be required to neutralize 27.2 mL of 0.491 M HNO3... Problem 70QAP: 70. What volume of a 0.500 M NaOH solution would be required to neutralize 40.0 mL of a 0.400 M... Problem 71QAP: 71. A sample of sodium hydrogen carbonate solid weighing 0.1015 g requires 47.21 ml. of a... Problem 72QAP: 72. The total acidity in water samples can be determined by neutralization with standard sodium... Problem 73QAP Problem 74QAP Problem 75QAP Problem 76QAP Problem 77QAP: 77. Explain why the equivalent weight of H2SO4 is half the molar mass of this substance. How many... Problem 78QAP Problem 79QAP Problem 80QAP Problem 81QAP Problem 82QAP Problem 83QAP Problem 84QAP Problem 85QAP: 85. How many milliliters of 0.50 N NaOH are required to neutralize exactly 15.0 mL of 0.35 N H2SO3? Problem 86QAP: 86. What volume of 0.104 N H2SO4is required to neutralize 15.2 mL of 0.152 N NaOH? What volume of... Problem 87QAP: 87. What volume of 0.151 N NaOH is required to neutralize 24.2 mL of 0.125 N H2SO4? What volume of... Problem 88QAP Problem 89AP: 89. A mixture is prepared by mixing 50.0 g of ethanol, 50.0 g of water, and 5.0 g of sugar. What is... Problem 90AP Problem 91AP: 91. Suppose 50.0 mL of 0.250 M CoCl2 solution is added to 25.0 mL of 0.350 M NiCl2 solution.... Problem 92AP Problem 93AP: 93. Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution,... Problem 94AP: 94. Baking soda (sodium hydrogen carbonate. NaHCO3) is often used to neutralize spills of acids on... Problem 95AP: 95. Many metal ions form insoluble sulfide compounds when a solution of the metal ion is treated... Problem 96AP Problem 97AP Problem 98AP Problem 99AP Problem 100AP Problem 101AP Problem 102AP: You mix 225.0 mL of a 2.5 M HCl solution with 150.0 mL of a 0.75 M HCl solution. What is the... Problem 103AP: A solution is 0.1% by mass calcium chloride. Therefore, 100. g of the solution contains g of calcium... Problem 104AP Problem 105AP Problem 106AP: A certain grade of steel is made by dissolving 5.0 g of carbon and 1.5 g of nickel per 100. g of... Problem 107AP Problem 108AP Problem 109AP Problem 110AP Problem 111AP: How many moles of each ion are present in 11.7 mL of 0.102 M Na3PO4solution? Problem 112AP Problem 113AP Problem 114AP Problem 115AP: Concentrated hydrochloric acid is made by pumping hydrogen chloride gas into distilled water, if... Problem 116AP Problem 117AP Problem 118AP Problem 119AP: If 10. g of AgNO3 is available, what volume of 0.25 M AgNO3 solution can be prepared? Problem 120AP Problem 121AP: Calcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion... Problem 122AP Problem 123AP: How many milliliters of 18.0 M H2SO4 are required to prepare 35.0 mL of 0.250 M solution? Problem 124AP: Consider the reaction between 1.0 L of 3.0 M AgNO3(aq) and 1.0 L of 1.0 M CuCl2(aq), according to... Problem 125AP: When 10. L of water is added to 3.0 L of 6.0 M H2SO4, what is the molarity of the resulting... Problem 126AP Problem 127AP: How many grams of Ba (NO3)2are required to precipitate all the sulfate ion present in 15.3 mL of... Problem 128AP Problem 129AP: What volume of 0.250 M HCI is required to neutralize each of the following solutions? a. 25.0 mL of... Problem 130AP Problem 131AP Problem 132AP Problem 133AP: How many milliliters of 0.105 M NaOH are required to neutralize exactly 14.2 mL of 0. 141 M H3PO4? Problem 134AP Problem 135AP Problem 136AP: Consider the reaction between 0.156 L of 0.105 M magnesium nitrate and 0.166 L of 0.106 M potassium... Problem 137CP Problem 138CP: A solution is prepared by dissolving 0.6706 g of oxalic acid (H2C2O4) in enough water to make 100.0... Problem 139CP: What volume of 0.100 M NaOH is required to precipitate all of the nickel(II) ions from 150.0 mL of a... Problem 140CP Problem 141CP: A 450.O-mL sample of a 0.257 M solution of silver nitrate is mixed with 400.00 mL of 0.200 M calcium... Problem 142CP: A 50.00-mL sample of aqueous Ca(OH)2 requires 34.66 mL of a 0.944 M nitric acid for neutralization.... Problem 143CP: When organic compounds containing sulfur are burned, sulfur dioxide is produced. The amount of... Problem 1CR Problem 2CR Problem 3CR Problem 4CR Problem 5CR Problem 6CR Problem 7CR Problem 8CR Problem 9CR Problem 10CR Problem 11CR Problem 12CR: Without consulting your textbook, list and explain the main postulates of the kinetic molecular... Problem 13CR: What does “STP’ stand for? What conditions correspond to STP? What is the volume occupied by one... Problem 14CR Problem 15CR Problem 16CR: Define the normal boiling point of water. Why does a sample of boiling water remain at the same... Problem 17CR: Are changes in state physical or chemical changes? Explain. What type of forces must be overcome to... Problem 18CR Problem 19CR Problem 20CR Problem 21CR: Define a crystalline solid. Describe in detail some important types of crystalline solids and name a... Problem 22CR: Define the bonding that exists in metals and how this model explains some of the unique physical... Problem 23CR Problem 24CR: Define a saturated solution. Does saturated mean the same thing as saying the solution is... Problem 25CR Problem 26CR: When a solution is diluted by adding additional solvent, the concentration of solute changes hut the... Problem 27CR Problem 28CR Problem 29CR Problem 30CR Problem 31CR Problem 32CR: When calcium carbonate is heated strongly, it evolves carbon dioxide gas. CaCO3(s)CaO(s)+CO2(g) 25 g... Problem 33CR: If an electric current is passed through molten sodium chloride, elemental chlorine gas is generated... Problem 34CR Problem 35CR Problem 36CR Problem 37CR Problem 38CR Problem 39CR Problem 40CR Problem 41CR Problem 42CR: 42. a. Fill in the following table as if it is a well plate and you are mixing two aqueous compounds... format_list_bulleted

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning




