
(a)
Interpretation:
A mechanism for each of the following transformations is to be suggested.
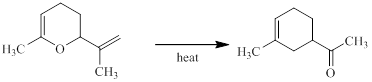
Concept introduction:
The Claisen rearrangement is a part of pericyclic reaction involving carbon-carbon bond formation. This reaction was discovered by Claisen and the reactions deals with the heating of allyl vinyl ether under thermal conditions to furnish an unsaturated carbonyl compound via [3, 3] sigmatropic rearrangement.
(b)
Interpretation:
A mechanism for each of the following transformations is to be suggested.

Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner.
(c)
Interpretation:
A mechanism for each of the following transformations is to be suggested.
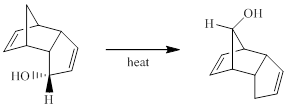
Concept introduction:
Generally, Sigmatropic reaction is referred as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- Show how you would synthesize the following compounds, starting with benzene or toluene and any necessary acyclicreagents. Assume para is the major product (and separable from ortho) in ortho, para mixtures. ethoxybenzenearrow_forwardProvide a plausible, stepwise mechanism to account for the following transformation. Use curved arrows to depict electron movement, and show all transition states/intermediates.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forward
- The transformation takes place via two sequential pericyclic reactions. Identify the two reactions and give a critical explanation whether the reactions are allowable or not. Explain the stereochemistry.arrow_forwardsynthesize the following compound given the starting material. Show all the steps, reagents and mechanisms. Do not skip anything. TAKE INTO ACCOUNT THE SHOWN STEREOCHEMISTRYarrow_forwardConsider the following chemical transformation:The transformation takes place via two sequential pericyclic reactions. Identify the two reactions and give a critical explanation whether the reactions are allowable or not. Explain the stereochemistryarrow_forward
- Show how the following starting materials are converted to the given product by a series of two pericyclic reactions. Account for the observed stereochemistry.arrow_forwardPropose a reasonable mechanism for each of the following transformations. Show clearly the structures of all the intermediates and electron flow.arrow_forwardAccount for any stereochemistry, major/minor products in the following reactions. Provide mechanistic explanations for your product(s).arrow_forward
