
Concept explainers
(a)
Interpretation:
An all-suprafacial [3, 3] sigmatropic rearrangement could in principle take place through either a chair-like or a boat-like transition state;

(a) According to the following results, two transition states are to be stated.
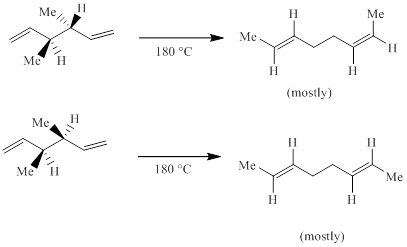
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner.
(b)
Interpretation:
When the terpene germacrone is distilled under reduced pressure at 165 °C, it is transformed to β-elemenone by a Cope rearrangement. The structure of germacrone, including its stereochemistry is to be deduced.
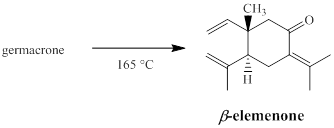
Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement. Notably, [3, 3] sigmatropic reaction of allyl vinyl ether is termed as Claisen rearrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- The existence of the NIH shift was established by determining the major product obtained from rearrangement of the following arene oxide, in which a hydrogen has been replaced by a deuterium. a. What would be the major product if the NIH shift occurs? b. What would be the major product if the carbocation forms phenol by losing H+ or D+, rather than by going through the NIH shift?arrow_forwardA key step in the synthesis of naproxen, an NSAID more commonly known by its brand name, Aleve (Section 3.9), is a coupling reaction of 2-bromo-6-methoxynaphthalene to form 2-methoxy-6-vinylnaphthalene. Show three different coupling reactions, and the required reagents, that could be used tocarry out this step.arrow_forwardPropose a plausible mechanism for the 1 to 2 conversion as shown, and explain the stereochemical result:arrow_forward
- Does the reaction of S-2-bromobutane with sodium hydroxide produce a racemic mixturearrow_forwardγ-Butyrolactone (C4H6O2, GBL) is a biologically inactive compound that is converted to the biologically active recreational drug GHB (Section 19.5) by a lactonase enzyme in the body. Since γ-butyrolactone is more fat soluble than GHB, it is more readily absorbed by tissues and thus produces a faster onset of physiological symptoms. γ-Butyrolactone shows an absorption in its IR spectrum at 1770 cm−1 and the following 1H NMR spectral data: 2.28 (multiplet, 2 H), 2.48 (triplet, 2 H), and 4.35 (triplet, 2 H) ppm. What is the structure of γ-butyrolactone?arrow_forward(2E,4Z,6Z,8E)-decatetraene has been cyclized to give 7,8-dimethy-1,3,5-cyclooctatriene. Predict the manner of ring-closure conrotatory or disrotatory for both thermal and photochemical reactions, and predict the stereochemistry of product in each case. Show how to get the answerarrow_forward
- When Br2 is added to buta-1,3-diene at -15 °C, the product mixture contains 60% ofproduct A and 40% of product B. When the same reaction takes place at 60 °C, theproduct ratio is 10% A and 90% B.If you had a solution of pure A, and its temperature were raised to 60 °C, what wouldyou expect to happen? Propose a mechanism to support your prediction.arrow_forwardProvide a reasonable arrow-pushing mechanism for Reaction 5b, and explain the the stereochemical outcome. 5d belowarrow_forwardwe know that ethers, such as diethyl ether and tetrahydrofuran, are quite resistant to the action of dilute acids and require hot concentrated HI or HBr for cleavage. However, acetals in which two ether groups are linked to the same carbon undergo hydrolysis readily, even in dilute aqueous acid. How do you account for this marked difference in chemical reactivity toward dilute aqueous acid between ethers and acetals?arrow_forward
- I. Molecular rearrangementsA) How are 1,2-hydride shifts in carbocation intermediates formed during SN1 or E1-type processes? How are carbocations formed by treating alcohols with water? How can you predict when a hydride shift will occur and how can you show it using arrow-pushing strategies?arrow_forwardExplain why bromopentane undergoes S N 2 substitutionreactions and 2 bromo 2 methyl pentane undergoes S N 1substitution reactions with ammonia. Clearly show the reactionmechanisms.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

