
Concept explainers
(a)
Interpretation:
In
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
In
Explanation of Solution
The structure of
This molecule contains seven carbon atoms. In case of the odd number of carbon atoms chain which is in conjugation, the molecular orbitals do not separate out into two equal halves in bonding and antibonding molecular orbitals. Along with that it gives rise to one molecular orbital whose energy is equal to that of
In
(b)
Interpretation:
Each molecular orbital of
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
The molecular orbitals
Explanation of Solution
In molecular orbital theory, the MO is said to be symmetric or anti-symmetric depending on the relative phase of the two terminal carbons. In symmetric MO, the peaks reflect across the reference plane into the peaks and troughs reflect into troughs. On the other hand, in antisymmetric MO, the peaks reflect into troughs and vice versa. According to the general principle, the even number molecular orbitals are antisymmetric and odd number molecular orbitals are symmetric. Therefore, the molecular orbitals
The molecular orbitals
(c)
Interpretation:
The carbon on which the positive charge is delocalized is to be stated. The explanation on the basis of resonance structures and molecular orbital arguments is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. Most of the organic structures cannot be represented using a single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. The delocalization of electrons results in the formation of resonance structure.
Answer to Problem 28.4P
The positive charge is delocalized over
Explanation of Solution
The resonance structure of
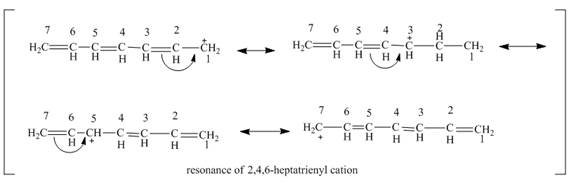
Figure 1
The resonance structures of
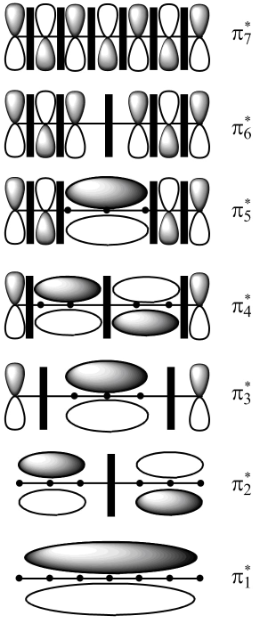
Figure 2
According to the general principle if the node is passing through the carbon then the positive charge is not present on that carbon. In this molecular orbital diagram, the node is passing through the
The positive charge is delocalized over
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- What is the degree of unsaturation of C8H10ClNO? a. 4 b. 5 c. 6 d. 7arrow_forwardDraw the structure of the one tertiary (3°) amine with molecular formula C5H13N that does not contain a 3-carbon chain. You do not have to consider stereochemistry. You do not have to explicitly draw H atomsarrow_forwardwhat is the bond line formula of C6H15N with respect to given IR spectra and NMRarrow_forward
- Methyl isocyanate (CH;NCO) is used in the manufacture of insecticides, such as carbaryl (Sevin®).Comment on the C-N bond distances within the molecule, Are they the same of different? Explain your answers.arrow_forwardEight alcohols have the formula C5H12O. Draw them. Which are chiral?arrow_forwardIdentify the structure of the compound. C8H19N MW=129arrow_forward
- Name the following Cd(C2H3O2)4arrow_forwardDraw the structure of the two tertiary (3°) amines with molecular formula C6H15N that contain a group with 3 carbon atoms in the largest group. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms.arrow_forwardThe molecule if C9 H10 O The IR spectra is provided, Identify the molecule, and functioal groups.arrow_forward
