
(a)
Interpretation:
When each of the compounds shown in Fig. P28.43 is heated in the presence of maleic anhydride, an inermediate is trapped as Diels-Alder adduct. The intermediate formed in each reaction, and its formation from the starting material are to be stated.
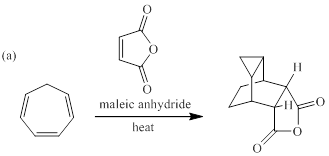
Concept introduction:
cycloaddition reaction can be referred as a reaction of two different π-electron systems to form a ring product. Notably, in this reaction two new sigma bonds are formed in product with the disappearance of two fewer π-bonds. In other words, cyclo addition reaction can be best described as the reaction of number of electrons involved. In [4+2] cycloaddition one component is having four electrons and other component is having two electrons.
(b)
Interpretation:
When each of the compounds shown in Fig. P28.43 is heated in the presence of maleic anhydride, an inermediate is trapped as Diels-Alder adduct. The intermediate formed in each reaction, and its formation from the starting material are to be stated.

Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement. Notably, [3, 3] sigmatropic reaction of allyl vinyl ether is termed as Claisen rearrangement.
(c)
Interpretation:
When each of the compounds shown in Fig. P28.43 is heated in the presence of maleic anhydride, an inermediate is trapped as Diels-Alder adduct. The intermediate formed in each reaction, and its formation from the starting material are to be stated.
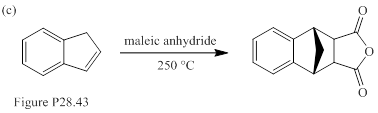
Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. Though, the position of π-bond is changed in Sigmatropic reaction, the total number of π-bonds remain unchanged. The sigma bond can be cleaved at the middle or at the end of the π-system. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement. Notably, [3, 3] sigmatropic reaction of allyl vinyl ether is termed as Claisen rearrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- Compare Electrophilic aromatic substitution reactivity to quinoline and isoquinoline. Please, explain with diagramsarrow_forwardBased on the given information, determine the diene and dienophile of the Diels-Alder reaction.arrow_forwardWhat happens to the stereochemistry during and SN2 reaction? Why? Provide a reaction to illustrate this.arrow_forward
- With reference to its molecular orbital diagram, state –with justification – how CO can act as a nucleophile, and whether it will attack its reacting partner through the carbon or oxygen atomarrow_forwardFor each of the following products, draw the structures of the diene and dienophile necessary to synthesize the compound in a Diels-Alder reaction.arrow_forwardWhat combination of diene and dienophile undergoes Diels-Alder reaction to give adduct?arrow_forward
- Draw the arrow pushing mechanism for the reaction of camphor to isoborneol. For each step on the mechanism give one DETAILED sentence on what is occurring and why. Examples: "Loss of water to form a tertiary carbocation", "nucleophilic attack of ____ to increase acidity of ____", "methyl shift of ____ to form ____", etc. Thank you!arrow_forwardIn the Friedel-Crafts alkylation of benzene, dialkylation is often a significant by-product. In the Friedel-Crafts acylation of benzene, diacylation is not a significant by-product. Why?arrow_forwardWith respect to the CIP rules, assign the groups high or low priority - also what is the overall stereochemistry of the double bond?arrow_forward
- A B ) Which of the above molecules (A or B) have a higher rate of reaction towards aromatic electrophilic reaction? Explain your answer.arrow_forwardGive a clear handwritten answer with explanation..give the SN2 mechanism of given bleow reactionarrow_forwardIn the Friedel-Crafts alkylation of benzene, dialkylation is often a significant by-product. In the Friedel-Crafts acylation of benzene, diacylation is not a significant by-product. Which of the following is the primary reason for this difference? a. Acyl cations are more difficult to make with Lewis acids b.Unlike acyl cations, carbocations can undergo rearrangements. c.Alkyl groups activate the ring to further substitution, acyl groups deactivate it. d. Alkyl groups activate the ring to further substitution, acyl groups deactivate itarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
