
Concept explainers
(a)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
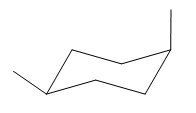
Explanation of Solution
The given compound is:
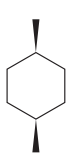
The cyclohexane ring has two methyl groups attached. Both these methyl groups are on the same side of the ring. Thus, they are cis to each other. These two methyl groups are the largest substituents on the ring and each of them is more stable in an equatorial position. Begin by drawing a chair conformation with a methyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
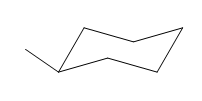
This methyl group is pointed up and the other methyl group must also point up, for them to be cis.

If the chair is flipped, the equatorial methyl group becomes axial and the axial methyl group becomes equatorial. In any case, one methyl group is axial and the other is equatorial.
Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has one substituent in axial position and other in equatorial position.
(b)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
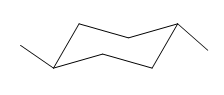
Explanation of Solution
The given compound is:
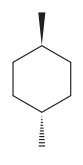
The cyclohexane ring has two methyl groups attached. Both these methyl groups are on the opposite side of the ring. Thus, they are trans to each other. These two methyl groups are the largest substituents on the ring and each of them is more stable in an equatorial position. Begin by drawing a chair conformation with a methyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:

This methyl group is pointed up and the other methyl group must point down, for them to be trans.

If the chair is flipped, both the equatorial methyl groups become axial. The chair conformation having both the methyl groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(c)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
Explanation of Solution
The given compound is:
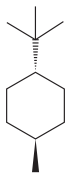
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:
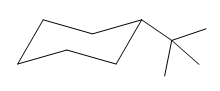
This tertiary butyl group is pointed down and the methyl group must point up, for them to be trans.
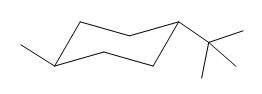
If the chair is flipped, both the equatorial groups become axial. The chair conformation having both the groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(d)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
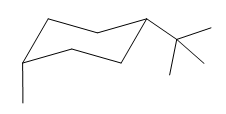
Explanation of Solution
The given compound is:
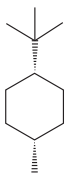
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:

This tertiary butyl group is pointed down and the methyl group must also point down, for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has the bulkier substituent in equatorial position and the other substituent in the axial position.
(e)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
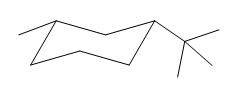
Explanation of Solution
The given compound is:
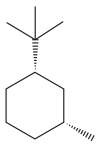
The cyclohexane ring has one methyl group and one tertiary butyl group attached. Both these substituents are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a dash bond, hence, it must point down in the chair conformation. It is shown below:

This tertiary butyl group is pointed down and the methyl group must also point down, for them to be cis.

If the chair is flipped, both the equatorial groups become axial. The chair conformation having both the alkyl groups in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
(f)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remains trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
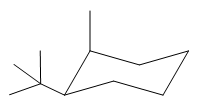
Explanation of Solution
The given compound is:
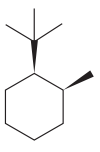
The cyclohexane ring has one tertiary butyl group and one methyl group attached. Both these groups are on the same side of the ring. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
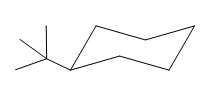
This tertiary butyl group is pointed up and the methyl group must also point up, for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has the bulkier substituent in equatorial position and the other substituent in axial position.
(g)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituents attached, then the conformation in which maximum substituents are in equatorial position is favored and is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip.
Answer to Problem 4.41P
The most stable conformation of the given molecule is:
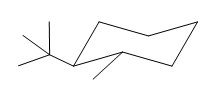
Explanation of Solution
The given compound is:
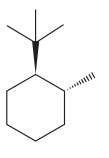
The cyclohexane ring has one tertiary butyl group and one methyl group attached. Both these groups are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring and it is more stable in an equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:

This tertiary butyl group is pointed up and the methyl group must also point down, for them to be trans.

If the chair is flipped, the equatorial tertiary butyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in equatorial position is more stable. Hence, the most stable chair conformation of the given molecule is:

The most stable conformation of the given molecule has both the substituents in the equatorial position.
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- Draw the more stable chair conformation for attached trisubstitutedcyclohexane.arrow_forwardIdentify the most stable chair conformation of the structure in the box shown in the attached picturearrow_forwardDetermine how many Gauche interactions are in the most stable chair conformation for each of the following structures.arrow_forward
- Draw the alternative chair conformation of this compound. Of the two chair conformations, which is more stable?arrow_forwardDraw both chair conformations for the following compoud.arrow_forwardDraw both chair conformers for the following molecule and circle the most stable conformation.arrow_forward
- Draw the equilibrium structure below that shows the two chair conformations of the following compound:arrow_forwardDraw the most stable chair conformation for each of the following disubstituted cyclohexanes.arrow_forwardWhich of the two products has the chair conformation of greater stability? Explain.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
