
The following diagram shows a simpli?ed version of how a refrigerator works:
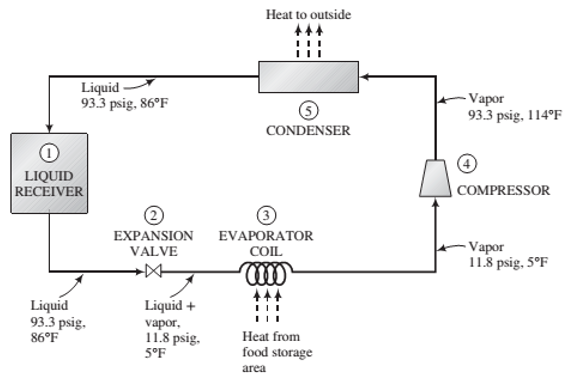
In a liquid receiver 1, a liquid refrigerant (any one of a number of halogenated hydrocarbons such as CCl2F3) is contained at high pressure and temperature. The liquid passes through an expansion valve 2, where it ?ashes to a low pressure, cooling to its boiling point at this pressure and partially evaporating. The liquid—vap0r mixture passes though an evaporator coil 3. Air from the food storage area circulates over the coil, and the heat absorbed by the evaporating refrigerant in the coil causes the air to cool. The cold refrigerant vapor emerging from the coil passes to a compressor 4, where it is brought back to a high pressure and in the process is raised to a high temperature. The hot vapor then passes through a condenser 5, where it is cooled and condensed at constant pressure. The air that absorbs the heat given up by the condensing ?uid is discharged outside the refrigerator, and the lique?ed refrigerant returns to the liquid receiver.
Suppose Refrigerant R−12 (the standard name for CCI2F2 undergoes this cycle at a circulation rate of 40 lbm/min, with the temperatures and pressures at the different points of the cycle being those shown on the ?ow diagram.
Saturated Fluid: 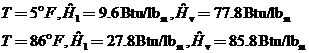
Superheated Vapor: 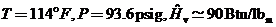
(a) Suppose the expansion valve operates adiabatically and 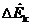 is negligible. Use an energy balance about the valve to calculate the fraction of the refrigerant that evaporates in this stage of the process.
is negligible. Use an energy balance about the valve to calculate the fraction of the refrigerant that evaporates in this stage of the process.
(b) Calculate the rate in Btu/min at which heat is transferred to the refrigerant that evaporates in the coil. (This is the useful cooling done in the system.)
(e) lf the heat loss in the condenser is 2500 Btu/min, how much horsepower must the compressor deliver to the system? (Use an overall energy balance to solve this problem.)
(d) You have just given a “What do engineers do?" talk at a middle-school career day, and one of the students in the audience asks if you can explain how a refrigerator works. Try to do it in terms that a bright l2-year-old student could understand.
(e) The manufacture of R−12 in the United States was banned as a result of the Montreal Protocol. Why?
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Fundamentals of Applied Electromagnetics (7th Edition)
Mechanics of Materials (10th Edition)
Software Engineering (10th Edition)
Data Mining for Business Analytics: Concepts, Techniques, and Applications with XLMiner
- Maple syrup sap is 3% sugar (sucrose) and 97% water bymass. Maple syrup is produced by heating the sap toevaporate a certain amount of the water. (a) Describe what happens to the composition and boilingpoint of the solution as evaporation takes place. (b) A rule of thumb among maple syrup producers is thatthe finished syrup should boil about 4 C higher than theoriginal sap being boiled. Explain the chemistry behindthis guideline. (c) If the finished product boils 4 C higher than the originalsap, calculate the concentration of sugar in the finalproduct. Assume that sugar is the only solute and theoperation is done at 1 atm pressure.arrow_forwardou place hot metal into a beaker of cold water. ol type='a'> Eventually what is true about the temperature of the metal compared to that of the water? Explain why this is true. i>Label this process as endothermic or exothermic if we consider the system to be the metal. Explain. the water. Explain.arrow_forwardDefine the joule in terms of SI base units.arrow_forward
- Are changes in state physical or chemical changes? Explain. What type of forces must be overcome to melt or vaporize a substance (are these forces intramolecular or intermolecular)? Define the molar heat of fusion and molar heat of vaporization. Why is the molar heat of vaporization of water so much larger than its molar heat of fusion? Why does the boiling point of a liquid vary with altitude?arrow_forwardFor each of the following pairs of solutions, select the solution for which solute solubility is greatest. a. Oxygen gas in water with P = 1 atm and T = 10C Oxygen gas in water with P = 1 atm and T = 20C b. Nitrogen gas in water with P = 2 atm and T = 50C Nitrogen gas in water with P = 1 atm and T = 70C c. Table salt in water with P = 1 atm and T = 40C Table salt in water with P = 1 atm and T = 70C d. Table sugar in water with P = 3 atm and T = 30C Table sugar in water with P = 1 atm and T = 80Carrow_forwardThe heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q, per mole of acid (or base) neutralized. Hneut for nitric acid is -52 kJ/mol HNO3. At 27.3C, 50.00 mL of 0.743M HNO3 is neutralized by 1.00 M Sr(OH)2 in a coffee-cup calorimeter. (a) How many mL of Sr(OH)2 were used in the neutralization? (b) What is the final temperature of the resulting solution? (Use the assumptions in Question 11.)arrow_forward
- How does the process described in the previous item relate to the system shown in Figure 16.4?arrow_forwardFor each of the following pairs of solutions, select the solution for which solute solubility is greatest. a. Ammonia gas in water with P = 1 atm and T = 50C Ammonia gas in water with P = 1 atm and T = 90C b. Carbon dioxide gas in water with P = 2 atm and T = 50C Carbon dioxide gas in water with P = 1 atm and T = 50C c. Table salt in water with P = 1 atm and T = 60C Table salt in water with P = 1 atm and T = 50C d. Table sugar in water with P = 2 atm and T = 40C Table sugar in water with P = 1 atm and T = 70Carrow_forwardSimple acids such as formic acid, HCOOH, and acetic acid, CH3COOH, are very soluble in water; however, fatty acids such as stearic acid, CH3(CH2)16COOH, and palmitic acid, CH3(CH2)14COOH, are water-insoluble. Based on what you know about the solubility of alcohols, explain the solubility of these organic acids.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





