
Saturated steam at a gauge pressure of 2.0 bar is to be used to heat a stream of ethane. The ethane enters a heat exchanger at 16°C and 1.5 bar gauge at a rate of 795 m3/min and is heated at constant pressure to 93°C. The steam condenses and leaves the exchanger as a liquid at 27°C. The speci?c enthalpy of ethane at the given pressure is 941 kJ/kg at 16°C and 1073 kJ/kg at 93°C.
(a) How much energy (kW) must be transferred to the ethane to heat it from 16°C to 93°C?
(b) Assuming that all the energy transferred from the steam goes to heat the ethane, at what rate in m3/s must steam be supplied to the exchanger? If the assumption is incorrect, would the calculated value be too high or too low?
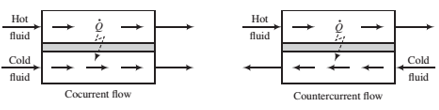
(c) Should the heat exchanger be set up for cocurrent or countercurrent ?ow (see the following schematic diagram)? Explain. (Hint: One of them will not work at all.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Differential Equations: Computing and Modeling (5th Edition), Edwards, Penney & Calvis
Database Concepts (8th Edition)
Experiencing MIS
Software Engineering (10th Edition)
- The gas A(g) (1 mole) undergoes a two-step process one after another as described below :i) The gas is expanded at 25°C and from 1 bar pressure against a constant pressure of 0.2 bar and the final volume of the gas is the twice the initial volume.ii) The gas is cooled down to -25°C at constant volume.Cv,m = 3/2 RCalculate ΔU, ΔH, q and w for the each step and for the entire processarrow_forwardCalculate the heat (q) and the work (w) in Joules, which the system exchanges with the return process in which the pressure is constant at 100 kPa. The initial temperature is 300 K and a final 500 K. The system contains 2 g of gas, which behaves in the state field the equation of an ideal gas and its molar heat capacity is cp, m = 43.0 J/g K. cv = 0.0346 J/g. Karrow_forwardThe synthesis of methanol from carbon monoxide and hydrogen is carried out in a continuous vapor-phase reactor at 5.00 atm absolute. The feed contains cO and H2 in stoichiometric proportion and enters the reactor at 25.0°C and 5.00 atm at a rate of 31.1 m³/h. The product stream emerges from the reactor at 157°C. The rate of heat transfer from the reactor is 21.0 kW. Calculate the fractional conversion (0 to 1) of carbon monoxide achieved and the volumetric flow rate (m3/h) of the product stream. f = Vout ! m3/harrow_forward
- = 1. A process has been proposed whereby an ideal gas is taken from P 10 bar and T = 300 K to P = 1 bar and T = 500 K in a closed system. During the process the system (ideal gas) does 1,000 kJ of work and receives 5,430 kJ of heat from the surroundings. The temperature of the surroundings is constant at 300 K. Ideal gas heat capacity: (b) Cp R = 3.6 +0.5 10-³T (T in K) Calculate the change in entropy of the gas.arrow_forwardAir with a relative humidity of 95% and temperature of 35°C is pumped into a chamber filled with a non-reactive porous material. The material has a porosity of 0.6 and is initially completely dry. After 15 minutes, air leaving the bed has temperature of 40°C and relative humidity of 5%. Complete this sentence: The air leaving the bed has a higher temperature than when it entered because: The latent heat of water in the gas phase was released by condensation onto the porous material The latent heat of water in the porous medium was released into the gas phase by a phase change. The latent heat of water in the gas phase was absorbed by evaporation The porous material was hot and transferred its energy to the air O Condensing water reacted with the porous material exothermically (that is, the reaction made heat)arrow_forwardIn a steam boiler, hot gases from a fire transfer heat to water which vapourizes at constant temperature.In certain case, the gases are cooled from 1100°C to 550°C while the water evaporates at 220°C. Thespecific heat of gases is 1.005 kJ/kg K, and the latent heat of water at 220°C is 1858.5 kJ/kg. All the heattransferred from the gases goes to the water. How much does the total entropy of the combined system ofgas and water increase as a result of irreversible heat transfer ? Obtain the result on the basis of 1 kg ofwater evaporated. If the temperature of the surroundings is 30°C find the increase in unavailable energydue to irreversible heat transfer.arrow_forward
- Nitroglycerin (C3H5N3O9), a liquid at room temperature (25 oC) and atmospheric pressure (1.01325 x 105 Pa) is used to relieve angina. It may undergo decomposition under these same conditions to form nitrogen, carbon dioxide, oxygen and water, with an enthalpy of decomposition of -1541 kJ mol-1The standard enthalpies of formation of water and carbon dioxide are -285.9 kJ mol-1 and -393.5 kJ mol-1 respectively Calculate the work done when 1.0 mole of nitroglycerin decomposes and additionally explain the significance of the sign.arrow_forwardNitroglycerin (C3H5N3O9), a liquid at room temperature (25 oC) and atmospheric pressure (1.01325 x 105 Pa) is used to relieve angina. It may undergo decomposition under these same conditions to form nitrogen, carbon dioxide, oxygen and water, with an enthalpy of decomposition of -1541 kJ mol-1.The standard enthalpies of formation of water and carbon dioxide are -285.9 kJ mol-1 and -393.5 kJ mol-1 respectively. Determine the standard enthalpy of formation of 1.0 mole of nitroglycerin.arrow_forward= 1. A process has been proposed whereby an ideal gas is taken from P 10 bar and T = 300 K to P= 1 bar and T 500 K in a closed system. During the process the system (ideal gas) does 1,000 kJ of work and receives 5,430 kJ of heat from the surroundings. The temperature of the surroundings is constant at 300 K. Cp Ideal gas heat capacity: = 3.6 + 0.5 * 10-³T (T in K) R (d) Does the process violate the second law of thermodynamics? Darrow_forward
- A closed system consisting of 2 lb of a gas undergoes a process during which the relation between pressure and volume is pV" = constant. The process begins with p₁= 15 lbf/in.2. v₁ = 1.25 ft3/lb and ends with p₂ = 60 lbf/in.2, v₂ = 0.5 ft3/lb. Determine (a) the volume, in ft², occupied by the gas at states 1 and 2 and (b) the value of n. Determine the volume, in ft, occupied by the gas at states 1 and 2. V₁= V₂ = Determine the value of n. n= 1 2 ft³ ft3arrow_forwardYou have analyzed an aqueous ammonia solution and find that it contains 30 wt% NH 3.(a) to determine the mass fraction of NH 3 in the vapor that would be in equilibrium with this solution in a closed flask at 1 atm and the corresponding system temperature.(b) If the liquid phase in Part (a) accounts for 90% of the total system mass, calculate the overall system composition and specific enthalpy using balances.arrow_forwardLiquid nitrogen is stored in 0.5-m3 metal tanks that are thoroughly insulated. Consider the process of filling an evacuated tank, initially at 295 K. It is attached to a line containing liquid nitrogen at its normal boiling point of 77.3 K and at a pressure of several bars. At this condition, its enthalpy is -120.8 kJ kg-1. When a valve in the line is opened, the nitrogen flowing into the tank at first evaporates in the process of cooling the tank. If the tank has a mass of 30 kg and the metal has a specific heat capacity of0.43 kJ kg-1 K-1, what mass of nitrogen must flow into the tank just to cool it to a temperature such that liquid nitrogen begins to accumulate in the tank? Assume that the nitrogen and the tank are always at the same temperature. The properties of saturated nitrogen vapor at several temperatures are given as follows:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





