
A mixture containing 65.0 mole% acetone (Ac) and the balance acetic acid (AA) is separated in a continuous distillation column at 1 atm. A ?owchart for the operation is as follows:
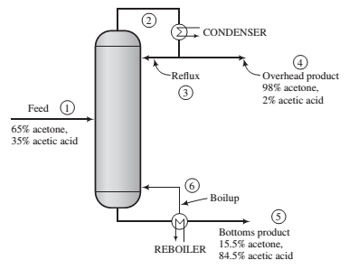
The stream from the top of the column is a vapor that passes though a condenser. The condensed liquid is divided into two equal streams: one is taken off as the overhead product (distillate) and the other (the re?ux) is returned to the column. The stream from the bottom of the column is a liquid that is partially vaporized in a reboiler. The liquid stream emerging from the reboiler is taken off as the bottoms product, and the vapor is returned to the column as boilup. Negligible heat is lost from the column, so that the only places in the system where external heat transfer takes place are the condenser and the reboiler.
|
|
||||
| Acetone | Acetic Acid | |||
| T(°C) |
|
|
|
|
| 56.8 | 0 | 7205 | 0 | 5723 |
| 63.0 | 205 | 7322 | 194 | 6807 |
| 67.5 | 354 | 7403 | 335 | 6884 |
| 98.7 | 1385 | 7946 | 1312 | 7420 |
- Taking 100 mol of feed as a basis, calculate the net heat requirement (cal) for the process. (You may neglect heats of mixing, although doing so for dissimilar liquids like acetone and acetic acid may introduce some error.)
- For the same basis, calculate the required heat input to the reboiler and the required heat removal from the condenser.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
Additional Science Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Objects First with Java: A Practical Introduction Using BlueJ (6th Edition)
Fundamentals of Applied Electromagnetics (7th Edition)
Fundamentals Of Thermodynamics
- Mail- Thomps 3 3 https://app.101edu.co 80 P Parchment Reg F3 $ 4 Write the balanced NET ionic equation for the reaction when AlCl3 and NaOH are mixed in aqueous solution. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 1 2 + 04- F4 Na set TW6D2PHI.pdf 2 % ( ) 5 3 4 03 □ F5 Unofficial Transcri 4 Question 15 of 40 ↑ NR O 6 5 05 0+ F6 6 UAFS Registra Graduation Applica MacBook Air & 7 2 2+ (s) (1) CI Al 7 8 9 0 09 口。 3+ 7 04+ F7 8 (g) (aq) • x H₂O 8 DII F8 H My UAFS-St Delete DD F9 M 0arrow_forwardEe.53.arrow_forwardThe need arises in a laboratory for 5000cm3 of an antifreeze solution consisting of 50mol% of HCl in water. What volumes of pure HCI and pure water at 25°c must be mixed to form 5000cm3 of antifreeze, also at 25°c partial molar volume of HCl and water in 50 mol% HCl solution and pure species volume, both at 25°c are: HCI : V 1=25.8956 cm³/mol , v1=75.8963 cm³/mol Water: 2 =42.5632 cm³/mol , v2=25.2362 cm3/mol.----- V --- (b). Prove Cp-Cy = R--------arrow_forward
- The proximate analysis (% air dried basis), the ultimate analysis (% d.m.m.f basis) and experimental gross calorific value (kcal/kg on air dried basis) is as follows: Proximate Ultimate analysis C.V. analysis % % Kcal/kg M VM А FC C HONS (exp. Value) 29 16 53 86 6 5 217200 Calculate its gross calorific value and net calorific value using (i) Goutal & (ii) Mazumdar correlations.arrow_forward1. Fresh air containing 4.00 mole % water vapor is to be cooled and dehumidified to a water content of 1.70 mole% H20. A stream of fresh air is combined with a recycle stream of previously dehumidified air and passed through the cooler. The blended stream entering the unit contains 2.30 mole% H2O. In the air conditioner, some of the water in the feed stream is condensed and removed as liquid. A fraction of the humidified air delivered to the cooler is recycled and the remainder is delivered to a room. Taking 100 mole of dehumidified air delivered to the room as a basis of calculation; calculate the moles of fresh feed, moles of water condensed, and moles of dehumidified air recycled. Recycle stream (R) Fresh air (F) Dehumidifier → 100 moles Water removed (Y)arrow_forward14. A solution of sulfuric acid in water, containing initially 73.13% by weight of H,SO, is diluted with water until the percentage of acid is 35.25 by weight. (a) What will be the integral heat of dilution when 100 g of the initial solution are diluted at 25°C? (b) What will be the differential heats of dilution of the acid and water under these conditions?arrow_forward
- Feed gas containing of 78.5mol % H₂, 21% of N₂ & 0.5% of Ar is mixed with recycle gas and enters a reactor where 15% N₂ is converted to NH3 as per the reaction. Ammonia from the exit of the reactor is completely separated from unconverted gases. To avoid the buildup of inerts, a small fraction (5%) of the unreacted gases purged and the balance recycled. USING ASPEN/HYSYS Draw the process flow sheet Product rate and Purge rate Basis:100mol/hrarrow_forward….arrow_forwardAn evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 19.6% wt K2SO4 The wet filter cake consists of solid K2SO4 crystals and 40 % wt K2SO4 solution, in a ratio 10 kg crystals/kg solution. The filte a 40% solution is recycled to join the fresh food. Of the water fed to the evaporator, 45% is evaporated The evaporator has a maximum capacity of 175 kg/hr water evaporales Calculate a) The maximam production rate of solid K2SO4 b) The rate at which fresh feed must be supplied to achieve this production rate c) The ratio kg recycle kg fresh feedarrow_forward
- The volume of HCI is 100 mL , mass of solid added is 1.008g, moles of solid 0.0276 Mol, mass of HCI is 100g, initial temperature is 21.8 degrees Celsius, Final temperature is 30.7 degrees Celsius, Calculate q rxn(= -mc Delta T of HCI),arrow_forwardA mixture combining 60 mL ethanol (C₂H6O) with 75 mL water (H₂O) is prepared at 280 K. The partial molar volumes of ethanol (E) and water (W) when mixed in these proportions (right column below) and in pure form (left column below) are ethanol water pure substance molar volume 58.2 mL/mol 18.00 mL/mol partial molar volume 55.3 mL/mol 17.7 mL/mol Determine the volume of the ethanol-water mixture. [Hint: You will need to determine the moles of each component.]arrow_forwardTo prepare a delicious banana milk on an industrial scale, there is an experimental process that consists of seven unit operations. A stream of banana (whose components are fruit, peel, and moisture) is fed to a peeler, where 99.9% of the peel is removed. This stream should become pulp, so it is mixed with an additive Q to avoid pulp oxidation. This mixture is then sent to an extruder press wherein a stream of a 50:50 mixture of additives Q and R is added. The resulting flow of 30 kg/h has a composition of 2% additive R and 5% additive Q. This flow is then fed to an agitated mixer. To the same mixer is fed a dairy stream of 250 kg/h that contains 70% milk (the rest is water) and a sweet aqueous stream that contains 30% sugar. For each 25 kg/h of dairy flow, 2 kg/h of the sweet flow is fed. The stream that leaves the agitated mixer has 5% fruit and 0.005% peel and enters a series of two thermal processes in which each operation allows 5% of the water and 2% of the milk to evaporate. The…arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





