
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 67IL
The photographs below (a) show what occurs when a solution of iron(III) nitrate is treated with a few drops of aqueous potassium thiocyanate. The nearly colorless iron(III) ion is converted to a red [Fe(H2O)5SCN)2+ ion. (This is a classic test for the presence of iron(III) ions in solution.)
[Fe(H2O)6]3+(aq) + SCN−(aq) ⇄ [Fe(H2O)5SCN]2+(aq) + H2O(ℓ)
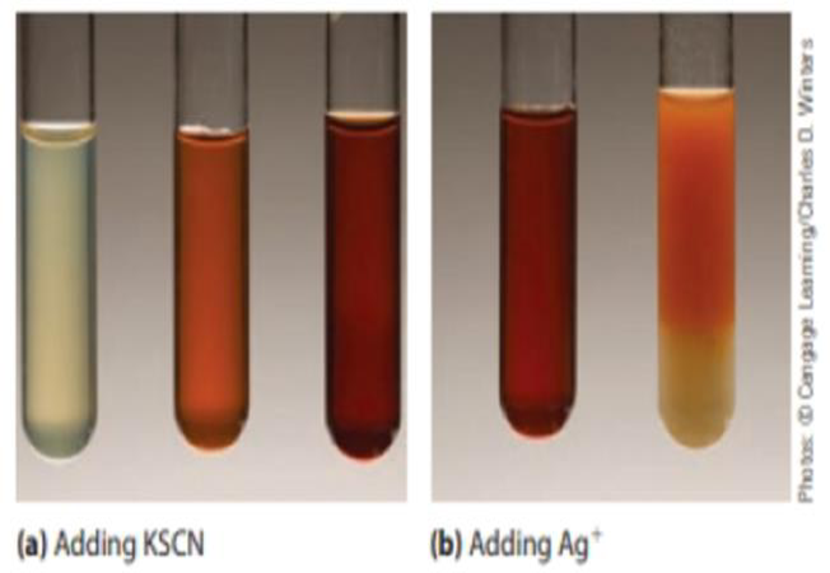
- (a) As more KSCN is added to the solution, the color becomes even more red. Explain this observation.
- (b) Silver ions form a white precipitate with SCN− ions. What would you observe on adding a few drops of aqueous silver nitrate to a red solution of [Fe(H2O)5 SCN]+ ions? Explain your observation.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Is there any evidence that the combination of Cu2+ and Fe3+ is a more effective catalyst than either alone?
The ficticious complex M(SCN)2 can be produced from an aqueous equilibrium in which the M2+ ion is mixed with thiocyanate ion. A solution containing initial concentrations of M2+ and SCN- are 0.000100 M and 0.000250 M respectively has an equilibrium concentration of M(SCN)2 of 3.00 x 10-5 M.
What are the equilibrium concentrations of the two starting ions and what is the value of the equilibrium constant?
[M2+]=___ x 10 __ M
[SCN-] = ___ x 10 __ M
Kc = ___ x 10 __ M
Use the data to the right for the next few questions: FeSCN+2 (Red) —> Fe+3 (Yellow) + SCN-1 (Colorless)
A. What will you observe if Fe(NO3)3 is added to the reaction? Explain your answer using Le Chatelier’s Principle and Q.
B. What will you observe if the solution is left out in an open container and some of the water evaporates? Explain your answer using Le Chatelier’s Principle and Q.
C. When the reaction is heated, a yellow color appears. Is the reaction endothermic or exothermic? Explain using Le Chatelier’s Principle.
Chapter 15 Solutions
Chemistry & Chemical Reactivity
Ch. 15.2 - Write the equilibrium constant expression for each...Ch. 15.2 - Answer the following questions regarding the...Ch. 15.3 - A solution is prepared by dissolving 0.050 mol of...Ch. 15.4 - At some temperature. Kc = 33 for the reaction...Ch. 15.4 - The decomposition of PCl5(g) to form PCl3(g) and...Ch. 15.5 - The conversion of oxygen to ozone has a very small...Ch. 15.6 - Equilibrium exists between butane and isobutane...Ch. 15.6 - Anhydrous ammonia is used directly as a...Ch. 15.6 - Prob. 1.2ACPCh. 15.6 - Freezing point depression is one means of...
Ch. 15.6 - Prob. 2.2ACPCh. 15.6 - A 0.64 g sample of the white crystalline dimer (4)...Ch. 15.6 - Predict whether the dissociation of the dimer to...Ch. 15.6 - Prob. 2.5ACPCh. 15 - Write equilibrium constant expressions for the...Ch. 15 - Write equilibrium constant expressions for the...Ch. 15 - Kc = 5.6 1012 at 500 K for the dissociation of...Ch. 15 - The reaction 2 NO2(g) N2O4(g) has an equilibrium...Ch. 15 - A mixture of SO2, O2, and SO3 at 1000 K contains...Ch. 15 - The equilibrium constant Kc, for the reaction 2...Ch. 15 - The reaction PCl5(g) PCl3(g) + Cl2(g) was...Ch. 15 - An equilibrium mixture of SO2, O2, and SO3 at a...Ch. 15 - The reaction C(s) + CO2(g) 2 CO(g) occurs at high...Ch. 15 - Hydrogen and carbon dioxide react at a high...Ch. 15 - A mixture of CO and Cl2 is placed in a reaction...Ch. 15 - You place 0.0300 mol of pure SO3 in an 8.00-L...Ch. 15 - The value of Kc for the interconversion of butane...Ch. 15 - Cyclohexane, C6H12, a hydrocarbon, can isomerize...Ch. 15 - The equilibrium constant for the dissociation of...Ch. 15 - The equilibrium constant, Kc, for the reaction...Ch. 15 - Carbonyl bromide decomposes to carbon monoxide and...Ch. 15 - Iodine dissolves in water, but its solubility in a...Ch. 15 - Which of the following correctly relates the...Ch. 15 - Which of the following correctly relates the...Ch. 15 - Consider the following equilibria involving SO2(g)...Ch. 15 - The equilibrium constant K for the reaction CO2(g)...Ch. 15 - Calculate K for the reaction SnO2(s) + 2 CO(g) ...Ch. 15 - Calculate K for the reaction Fe(s) + H2O(g) ...Ch. 15 - Relationship of Kc and Kp: (a) Kp for the...Ch. 15 - Relationship of Kc and Kp: (a) The equilibrium...Ch. 15 - Dinitrogen trioxide decomposes to NO and NO2, in...Ch. 15 - Kp for the following reaction is 0.16 at 25 C: 2...Ch. 15 - Consider the isomerization of butane with an...Ch. 15 - The decomposition of NH4HS NH4HS(s) NH3(g) +...Ch. 15 - Suppose 0.086 mol of Br2 is placed in a 1.26-L...Ch. 15 - The equilibrium constant for the reaction N2(g) +...Ch. 15 - Kp for the formation of phosgene, COCl2, is 6.5 ...Ch. 15 - The equilibrium constant, Kc, for the following...Ch. 15 - Carbon tetrachloride can be produced by the...Ch. 15 - Equal numbers of moles of H2 gas and I2 vapor are...Ch. 15 - The equilibrium constant for the butane isobutane...Ch. 15 - At 2300 K the equilibrium constant for the...Ch. 15 - Which of the following correctly relates the two...Ch. 15 - Consider the following equilibrium: COBr2(g) ...Ch. 15 - Heating a metal carbonate leads to decomposition....Ch. 15 - Phosphorus pentachloride decomposes at elevated...Ch. 15 - Ammonium hydrogen sulfide decomposes on heating....Ch. 15 - Ammonium iodide dissociates reversibly to ammonia...Ch. 15 - When solid ammonium carbamate sublimes, it...Ch. 15 - In the gas phase, acetic acid exists as an...Ch. 15 - Assume 3.60 mol of ammonia is placed in a 2.00-L...Ch. 15 - The total pressure for a mixture of N2O4 and NO2...Ch. 15 - Kc for the decomposition of ammonium hydrogen...Ch. 15 - Prob. 52GQCh. 15 - A 15-L flask at 300 K contains 6.44 g of a mixture...Ch. 15 - Lanthanum oxalate decomposes when heated to...Ch. 15 - The reaction of hydrogen and iodine to give...Ch. 15 - Sulfuryl chloride, SO2Cl2 is used as a reagent in...Ch. 15 - Hemoglobin (Hb) can form a complex with both O2...Ch. 15 - Limestone decomposes at high temperatures....Ch. 15 - At 1800 K, oxygen dissociates very slightly into...Ch. 15 - Nitrosyl bromide, NOBr, dissociates readily at...Ch. 15 - A Boric acid and glycerin form a complex...Ch. 15 - The dissociation of calcium carbonate has an...Ch. 15 - A sample of N2O4 gas with a pressure of 1.00 atm...Ch. 15 - Prob. 64GQCh. 15 - The photograph below shows what occurs when a...Ch. 15 - The photographs below (a) show what occurs when a...Ch. 15 - Decide whether each of the following statements is...Ch. 15 - Neither PbCl2 nor PbF2 is appreciably soluble in...Ch. 15 - Characterize each of the following as product- or...Ch. 15 - The size of a flask containing colorless N2O4(g)...Ch. 15 - Describe an experiment that would allow you to...Ch. 15 - The chapter opening photograph (page 670) showed...Ch. 15 - Suppose a tank initially contains H2S at a...Ch. 15 - Pure PCl5 gas is placed in a 2.00-L flask. After...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
16.43 The following pictures represent solutions at various stages in thetitration of a weak diprotic acid with...
Chemistry (7th Edition)
The structural formula of 1, 2-dimethylbenzene needs to be drawn. Concept introduction: The ring structures of ...
Chemistry: Matter and Change
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the K expression for the following reaction: CO(g)+NO2(g)⇌CO2(g)+NO(g)arrow_forwardDescribe the reaction Cu(OH)2 → CuO + H2Oarrow_forwardThe formation of the iron(III) thiocyanate complex ion is an exothermic equilibrium system. For each change to the system, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. Fe3+(aq) ++ SCN−(aq) ↽−−⇀↽−−⇀ Fe(SCN)2+(aq) increasing the concentration of Fe3+ decreasing the concentration of Fe(SCN)2 increasing the temperature of the system Answer Bank ↑↑ ↓↓arrow_forward
- Consider the insoluble compound cobalt(II) hydroxide , Co(OH)2 . The cobalt(II) ion also forms a complex with ammonia . Write a balanced net ionic equation to show why the solubility of Co(OH)2 (s) increases in the presence of ammonia and calculate the equilibrium constant for this reaction.For Co(NH3)62+ , Kf = 7.7×104 . Be sure to specify states such as (aq) or (s). + + K =arrow_forwardIf Kp is the equilibrium constant for the reaction 2 A (g) + B (g) ⇌ 3 C (g). What is the equilibrium constant (in terms of Kp) for the reaction 6 A (g) + 3 B (g) ⇌ 9 C (g)?arrow_forwardGiven the following reactions and their equilibrium constants, H2O(g) + CO(g) ↽−−⇀↽−−⇀ H2(g) + CO2(g) K = 1.6 FeO(s) + CO(g) ↽−−⇀↽−−⇀ Fe(s) + CO2(g) K = 0.67 calculate K for the reaction Fe(s) + H2O(g) ↽−−⇀↽−−⇀ FeO(s) + H2(g)arrow_forward
- The formation of the iron(III) thiocyanate complex ion is an exothermic equilibrium system. For each change to the system, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. increasing the concentration of Fe3+ decreasing the concentration of Fe(SCN)2+ increasing the temperature of the systemarrow_forwardConsider the following equilibrium reaction: A (aq) + B (aq) ⇌2 C (s) + D (g) + 16 cal d)Under what conditions of temperature and pressure will the maximum yield of product be produced?arrow_forwardThe reduction of iron(III) oxide to iron during steel-making can be summarized by this sequence of reactions: 2C(s)+O2(g) ⇌ 2CO(g) K1 Fe2O3(s)+3CO(g) ⇌ 2Fe(l)+3CO2(g) K2 The net reaction is: 2Fe2O3(s)+6C(s)+3O2(g) ⇌ 4Fe(l)+6CO2(g) K Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K1 and K2.arrow_forward
- The equilibrium constant, Kp, for the following reaction is 0.215 at 673 K.NH4I(s) NH3(g) + HI(g)If ΔH° for this reaction is 182 kJ, what is the value of Kp at 764 K?Kp = ______arrow_forwardThe overall reaction for the corrosion of iron (rusting) by oxygen is 4Fe(s) + 3O2 --> 2Fe2O3 (s) Calculate ΔGo for the reaction using ΔG = ΔH – TΔS. ΔG = -1484.5 kJ Calculate the equilibrium constant using ΔGo = -RT ln K. K = 1.58 x 1060 What does this mean for the fate of iron in our environment?arrow_forwardDescribe the reaction Cu(OH)2(s) ->arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY