
Biochemistry: The Molecular Basis of Life
6th Edition
ISBN: 9780190209896
Author: Trudy McKee, James R. McKee
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 62TQ
Summary Introduction
To review:
The transition state for each step involved in the exothermic hydrolysis of tertiary-butyl chloride, resulting in t (tertiary)-butyl alcohol and chloride ions. The reactions are given as follows:
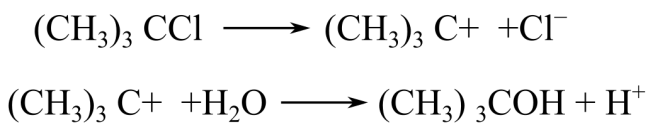
Introduction:
A biochemical reaction follows the same rules as followed by a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Why was dinitrophenol once used as a diet drug?
What would be the fate of oxaloacetate if there were sufficient fluoroacetate present?
What Are the Anaplerotic, or “Filling Up,”Reactions?
Chapter 6 Solutions
Biochemistry: The Molecular Basis of Life
Ch. 6 - Prob. 1QCh. 6 - Prob. 2QCh. 6 - Prob. 3QCh. 6 - Prob. 4QCh. 6 - Prob. 5QCh. 6 - Prob. 6QCh. 6 - Prob. 7QCh. 6 - Prob. 8QCh. 6 - Prob. 9QCh. 6 - Prob. 1RQ
Ch. 6 - Prob. 2RQCh. 6 - Prob. 3RQCh. 6 - Prob. 4RQCh. 6 - Prob. 5RQCh. 6 - Prob. 6RQCh. 6 - Prob. 7RQCh. 6 - Prob. 8RQCh. 6 - Prob. 9RQCh. 6 - Prob. 10RQCh. 6 - Prob. 11RQCh. 6 - Prob. 12RQCh. 6 - Prob. 13RQCh. 6 - Prob. 14RQCh. 6 - Prob. 15RQCh. 6 - Prob. 16RQCh. 6 - Prob. 17RQCh. 6 - Prob. 18RQCh. 6 - Prob. 19RQCh. 6 - Prob. 20RQCh. 6 - Prob. 21RQCh. 6 - Prob. 22RQCh. 6 - Prob. 23RQCh. 6 - Prob. 24RQCh. 6 - Prob. 25RQCh. 6 - Prob. 26RQCh. 6 - Prob. 27RQCh. 6 - Prob. 28RQCh. 6 - Prob. 29RQCh. 6 - Prob. 30RQCh. 6 - Prob. 31RQCh. 6 - Prob. 32RQCh. 6 - Prob. 33RQCh. 6 - Prob. 34RQCh. 6 - Prob. 35RQCh. 6 - Prob. 36RQCh. 6 - Prob. 37RQCh. 6 - Prob. 38RQCh. 6 - Prob. 39RQCh. 6 - Prob. 40RQCh. 6 - Prob. 41RQCh. 6 - Prob. 42RQCh. 6 - Prob. 43FBCh. 6 - Prob. 44FBCh. 6 - Prob. 45FBCh. 6 - Prob. 46FBCh. 6 - Prob. 47FBCh. 6 - Prob. 48FBCh. 6 - Prob. 49FBCh. 6 - Prob. 50FBCh. 6 - Prob. 51FBCh. 6 - Prob. 52FBCh. 6 - Prob. 53SACh. 6 - Prob. 54SACh. 6 - Prob. 55SACh. 6 - Prob. 56SACh. 6 - Prob. 57SACh. 6 - Prob. 58TQCh. 6 - Prob. 59TQCh. 6 - Prob. 60TQCh. 6 - Prob. 61TQCh. 6 - Prob. 62TQCh. 6 - Prob. 63TQCh. 6 - Prob. 64TQCh. 6 - Prob. 65TQCh. 6 - Prob. 66TQCh. 6 - Prob. 67TQCh. 6 - Prob. 68TQCh. 6 - Prob. 69TQCh. 6 - Prob. 70TQCh. 6 - Prob. 71TQCh. 6 - Prob. 72TQCh. 6 - Prob. 73TQCh. 6 - Prob. 74TQCh. 6 - Prob. 75TQCh. 6 - Prob. 76TQCh. 6 - Prob. 77TQCh. 6 - Prob. 78TQCh. 6 - Prob. 79TQCh. 6 - Prob. 80TQCh. 6 - Prob. 81TQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON