
To review:
The type of inhibition by A and B inhibitor and the kind of affinity they possess for ES versus E.
Introduction:
Enzyme inhibitors are the molecules that are able to bind to enzyme and reduce the activity. Enzyme inhibitors work by several ways such as preventing the substrate from entering the active site of an enzyme or hinder in catalyzing the reaction. There are three types of enzyme inhibitors, namely, competitive, noncompetitive, and uncompetitive.
Explanation of Solution
The velocity of the reaction at different substrate concentrations in presence of A inhibitor and B inhibitor are given in the question. For calculating, the Vmax (maximum
| [S] in mM (millimolar) |
V | |
|
| 1.3 | 1.17 | 0.76 | 0.855 |
| 2.6 | 2.10 | 0.38 | 0.476 |
| 6.5 | 4.00 | 0.15 | 0.25 |
| 13.0 | 5.70 | 0.077 | 0.175 |
| 26.0 | 7.20 | 0.038 | 0.139 |
The following table shows the values of [S], V,
| [S] in mM (millimolar) |
V | |
|
| 1.3 | 0.62 | 0.76 | 1.613 |
| 2.6 | 1.42 | 0.38 | 0.704 |
| 6.5 | 2.65 | 0.15 | 0.377 |
| 13.0 | 3.12 | 0.077 | 0.321 |
| 26.0 | 3.58 | 0.038 | 0.28 |
Plotting the graph between
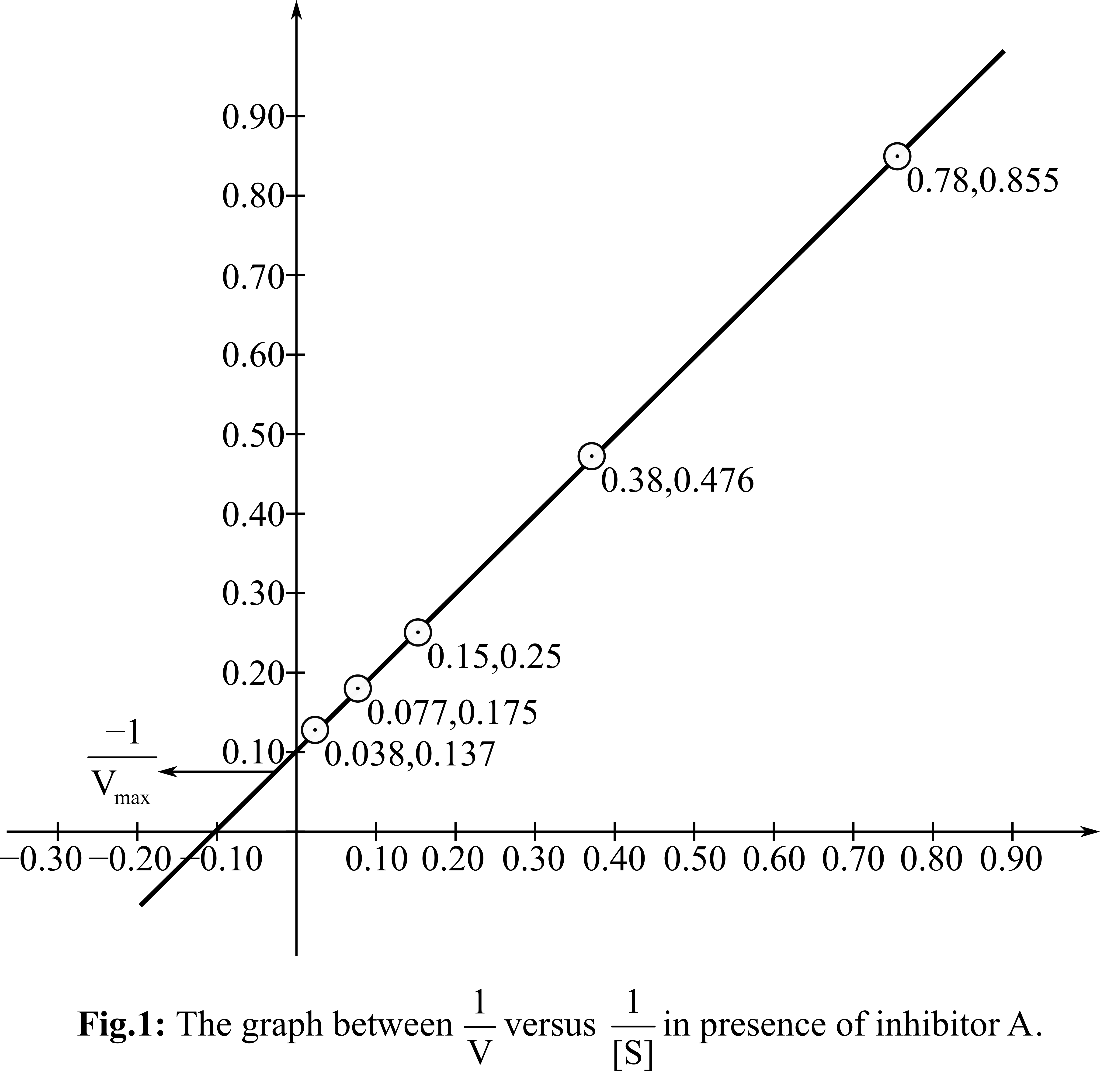
According, to the Michelis Menten equation, the value of y-intercept represents
From the graph, it can be observed that the value of y-intercept is 0.10 mM-1 sec. y-Intercept represents inverse of Vmax.
KM can be calculated by putting the value of Vmax in the equation Slope=KM/Vmax
So, the KM for the reaction is 9.8and the Vmax is 10 mM. sec-1. In presence of inhibitor A, KM is changed but Vmax is unchanged. So, it is a competitive inhibitor.
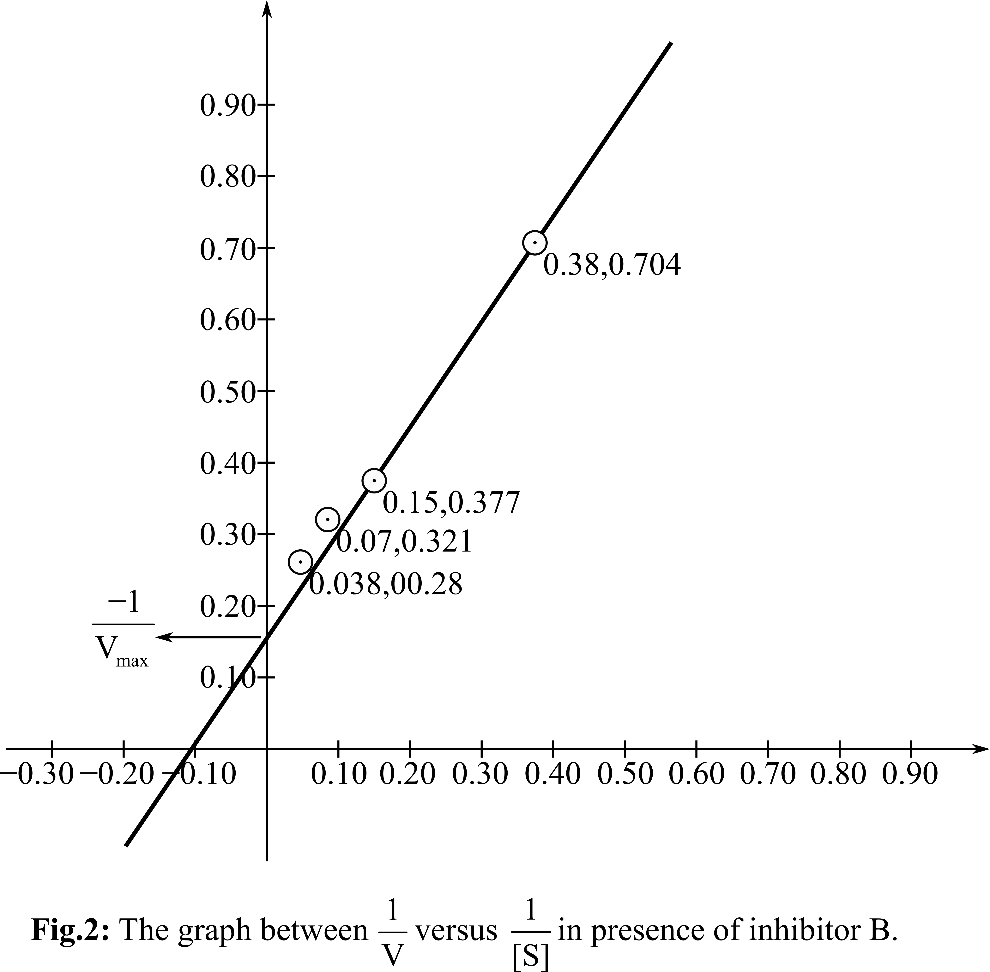
According, to the Michelis Menten equation, the value of y-intercept represents
From the graph, it can be observed that the value of y-intercept is 0.25 mM-1 sec. y-Intercept represents inverse of Vmax.
KM can be calculated by putting the value of Vmax in the equation Slope=KM/Vmax
So, the KM for the reaction is 5.68 and the Vmax is4 mM. sec-1. In presence of inhibitor B, KM andVmax both are changed. So, it is anuncompetitive inhibitor. A inhibitor is a competitive inhibitor so it can bind to free enzyme and has no affinity for ES. B inhibitor is an uncompetitive inhibitor, so it can bind to ES as well as E.
Therefore, it can be concluded that inhibitor A is competitive as Vmax remain unchanged whereas, inhibitor B is uncompetitive inhibitor. A can bind to free enzyme and B can bind to E as well as ES.
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry: The Molecular Basis of Life
- This is a conjectural question: If the reactive part of coenzyme A is the thioester, why is the molecule socomplicated?arrow_forwardtrue or false: Glutamine PRPP amidotransferase is regulated via product inhibition.arrow_forwardWhy is citrate an appropriate inhibitor of phosphofructokinase?arrow_forward
- Why does the enzyme reaction for chymotrypsin proceed in two phases?arrow_forwardWhy is trypsin an unusual name for an enzyme? (What is the convention for enzyme names?) .arrow_forwardThe following phenylboronate is a competitive inhibitor of a chymotrypsin-like enzyme. Unlike carbon, boron is quite happy to be bonded to three oxygens (and one carbon)—explain why this feature makes it a much better inhibitor than the corresponding carboxylic acid (which is quite ineffective).arrow_forward
- In order to function as an oxidative phosphorylation uncoupler, 2,4-dinitrophenol must act catalytically, not stoichiometrically. What does this mean? Identify and discuss an important implication of this conclusion.arrow_forwardEnzymes are stereochemically specific; that is, they oftenconvert only one stereoisomeric form of substrate intoproduct. Why is such specificity inherent in theirstructure?arrow_forwardWhat is ornithine transcarbamylase (OTC)deficiency ?arrow_forward
- The sedimentation value of aspartate transcarbamoylase decreases when the enzyme switches to the R state. On the basis of the allosteric properties of the enzyme, explain why the sedimentation value decreases.arrow_forwardGlycogen phosphorylase is an example of an enzyme that is regulated both by allosteric control and by covalent modification. Explain how this enzyme is regulated by both allosteric control and covalent modificationarrow_forwardWhat does this important observation imply about the relation between the amino acid sequence of insulin and its three-dimensional structure?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





